(UroToday.com) As a portion of the European Society for Medical Oncology (ESMO) Annual Congress, an Educational Session focused on precision management of patients with prostate cancer was held. In this context, Dr. Mark Rubin presented the role of molecular pathology to guide treatment for patients with advanced prostate cancer.
He began by emphasizing that insights into how molecular alternations that occur in the tumorigenesis pathway may influence therapy, it is particularly important to consider those that may confer treatment resistance.
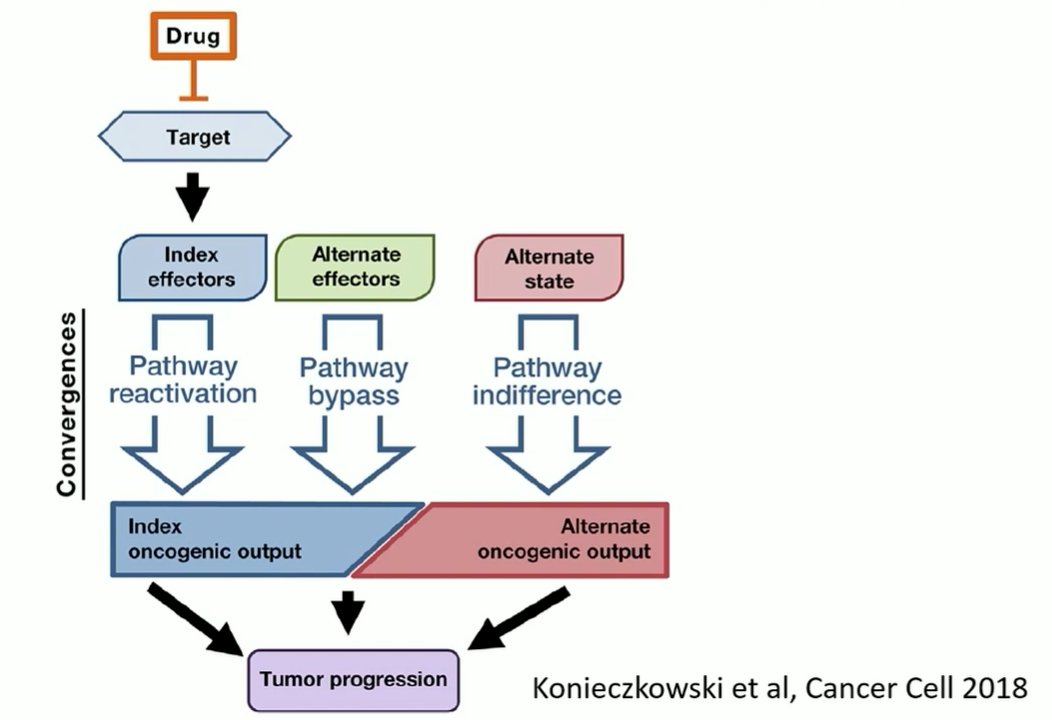
In the context of prostate cancer, androgen axis signaling inhibition remains a major line of therapy, even for patients with castration resistant disease. In particular, clinically utilized approaches seek to lower testosterone or inhibit the androgen receptor. However, molecular alterations in the androgen receptor may confer treatment resistance. They may therefore be used molecular biomarkers to assess for disease responsiveness. Further, in addition to these alterations in the primary driving pathway for prostate cancer, other alterations may occur leading to alternate pathway activation (eg. WNT5A) which confer a different, neuroendocrine phenotype as a result of transdifferentiation.
He then focused on the question of homologous recombination repair defects and mismatch repair alterations. In the context of advanced prostate cancer, 5% of patients may have microsatellite instability or mismatch repair alterations which render them sensitive to pembrolizumab, 10% will be expected to have germline DNA repair mutations, and 20% will have DNA repair alterations at either the somatic or germline level which may sensitize patients to PARP inhibition or platinum-based chemotherapy treatment.
Prior to moving forward, Dr. Rubin emphasized two important definitions, distinguishing between prognostic biomarkers and predictive biomarkers. While prognostic biomarkers provide some assessment of the risk of a clinical event (often disease progression or death), predictive biomarkers assess whether a patient is like to respond to exposure (often treatment intervention).

In terms of the derivation of samples for testing, Dr. Rubin highlighted that buccal samples may be used for germline testing, tumor samples (from biopsy typically) may be used for genomic sequencing or transcriptomic sequencing, and blood samples may be used for the detection of cfDNA, CTCs, or tumor-related metabolites.
Dr. Rubin then focussed on the question of homologous recombination repair (HRR). While initial work focused on germline BRCA mutations, and the role of PARP inhibition, a similar treatment phenomenon is present among those with somatic mutations. The principle of PARP inhibition is predicated on the idea of synthetic lethality in which cells may survive with either HRR mutations or PARP inhibition, but not both.
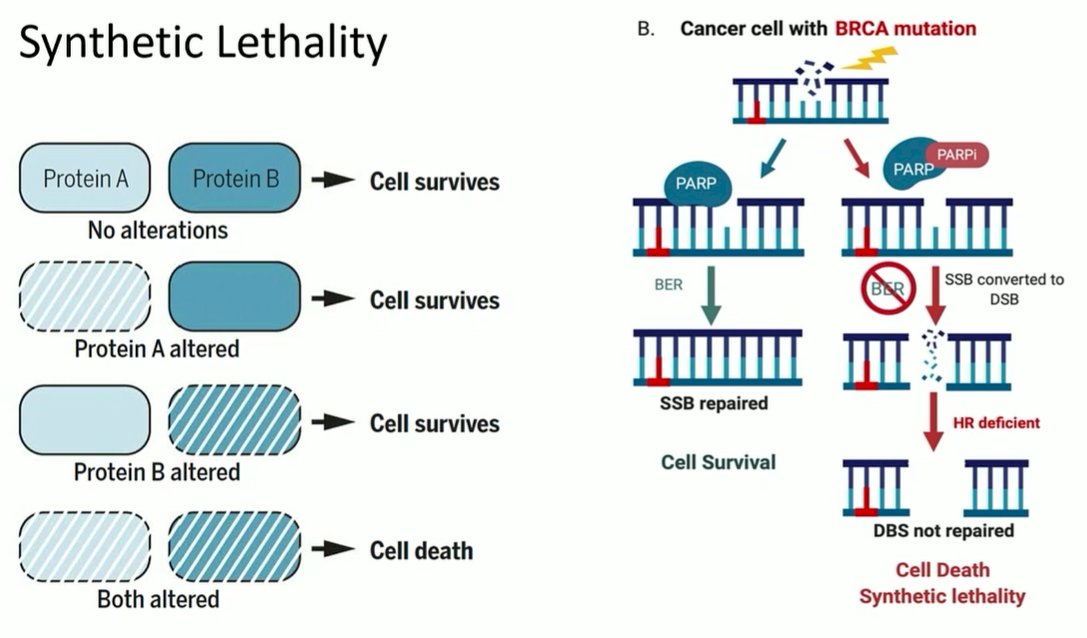
In the context of advanced prostate cancer, this is important as relatively large proportions of patients with the disease have relevant mutations in HRR genes including BRCA1 and BRCA2. As a result, targeting this subset of patients with PARP inhibition is clinically relevant. The TOPARP trial was the first data demonstrating a differential response to PARP inhibition among prostate cancer patients with BRCA mutations. In terms of translating to clinical practice, the PROfound trial provides randomized data supporting the use of biomarker-driven treatment approaches for patients with advanced prostate cancer, and supporting the use of biomarker selection for advanced prostate cancer treatment.
In addition to those mutations which are common, Dr. Rubin emphasized the “long tail” of prostate cancer mutations. These are infrequent mutations. However, they may be clinically relevant and thus require large samples to identify these.
Further, he emphasized that there may be resistance to these novel approaches. In the context of PARP inhibition, a variety of mechanisms may lead to resistance, including reversion mutations, DDR rewiring, a drug-specific mechanism, or transporter overexpression.
Dr. Rubin then transitioned to discussing microsatellite instability and mismatch repair in advanced prostate cancer. Overall, this is seen in approximately 5-8% of patients with advanced prostate cancer. While this is a relatively small sample, there remains heterogeneity in treatment response within this group. Thus, the biomarker is predictively imperfect.
Outside prostate cancer, he highlighted recent work examining whole genome sequencing, including examination of DNA scar and a variety of underlying causes. This has now been incorporated into ESMO guidelines.
Dr. Rubin also noted that rates of DNA alterations may differ on the basis of histology: patients with ductal adenocarcinoma appear to have particularly high rates of DNA repair gene mutations. Additionally, there is evidence that microsatellite instability may differ between tumor types. Thus, the application of a general panel may not be appropriate and we may need to develop tumor-specific approaches.
Dr. Rubin then circled back to where he began, emphasizing that DNA repair mutations and mismatch repair/microsatellite instability comprise approximately one-quarter of patients with advanced prostate cancer. Thus, the remaining three-quarters have some other alterations underlying their pathogenesis. A number of novel treatment approaches may be able to target these factors. In particular, he highlighted numerous ongoing trials examining the role of PSMA-targeted theranostics. Embedded in many of these trials are correlative biomarker analyses, including assessment of CTCs.
Highlighting the example of a primary prostate tumor with multiple lesions, he emphasized the importance of overcoming heterogeneity (both within a lesion and between lesions within a patient) to allow for an accurate understanding of the underlying tumor biology. Thus, conventional biopsy may not provide as useful information as assessment of liquid biomarkers such as cfDNA or CTCs.


