(UroToday.com) In the on-demand poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Holger Palmedo reported results of the PARABO cohort (NCT02398526), a prospective, observational, noninterventional, single-arm study evaluating pain efficacy in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with Radium-223 in German real-life nuclear medicine settings.
In the ALSYMPCA trial, radium-223 demonstrated a significant survival advantage, favorable safety profile, and delayed opioid use and external beam radiotherapy.
In this observational cohort, the authors assessed the primary outcome of pain response, defined as a ≥2 point improvement in Brief Pain Inventory short form (BPI-SF) worst pain score in pts with baseline worst pain >1. The authors report pain response in the overall population and by the extent of disease (EOD; ≤20 vs >20 lesions), opioid use, and Ra-223 cycles received (5–6 vs ≤4). Patient-reported outcomes were reported using the BPI-SF component scores which were assessed at each visit.
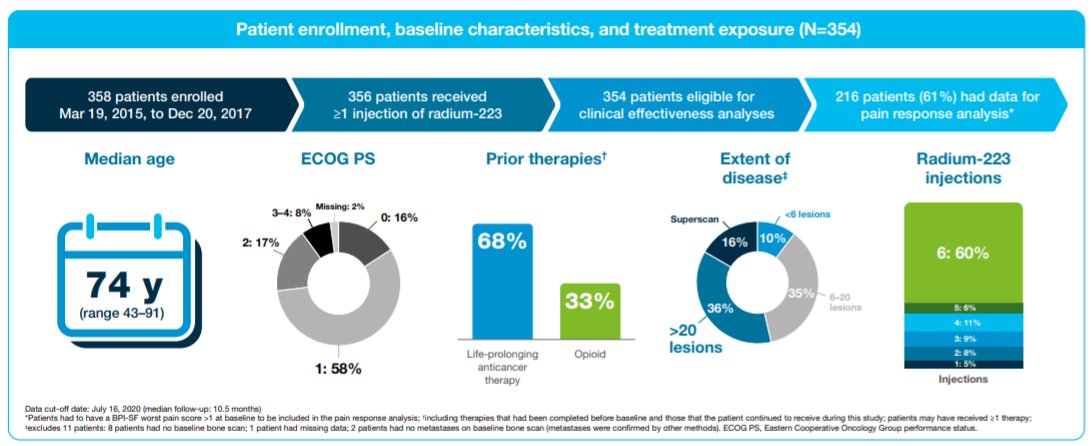
As of a data cutoff of July 16, 2020, the analytic cohort includes 354 patients who started Radium-223 between Mar 2015 and Dec 2017. At baseline, 260 patients (73%) had Eastern Cooperative Oncology Group performance status 0 or 1. In terms of disease burden, 37 patients (10%) had <6 metastatic lesions, 124 (35%) had 6–20 lesions, 127 (36%) had >20 lesions, and 55 (15%) had a super scan. Additionally, 242 patients (68%) had received prior systemic life-prolonging therapy (median 2). In total, 169 patients (48%) used opioids at any time (116 used opioids before/at baseline, 53 started opioids during Radium-223 therapy).
Overall, patients received a median of 6 Radium-223 injections (range 1–6), with the majority (n=236, 67%) receiving 5–6 injections and 118 (33%) having ≤4 injections.
Among the 216 patients with baseline worst pain >1, 128 (59%) had a clinically meaningful pain response. Notably, this effect was similar in patients with ≤20 lesions (60%) vs 59% >20 lesions (59%). Further, the effect was similar in those with opioid use (65%) vs non-opioid use (54%). However, patients who received 5–6 Radium-223 injections were somewhat more likely to have a clinically meaningful pain response (67%) compared to those who received ≤4 injections (43%).
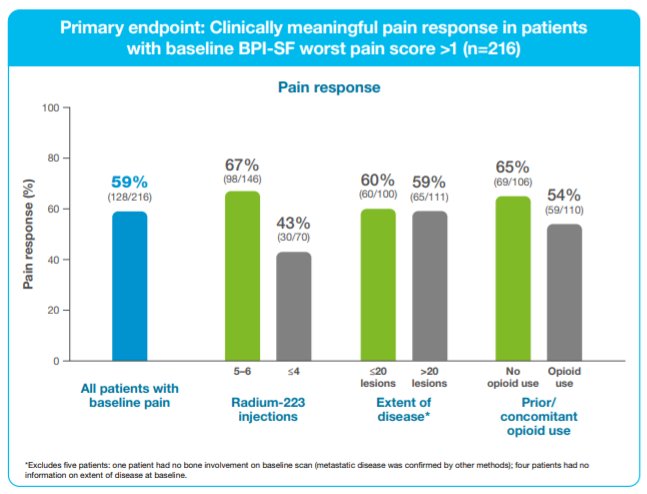
Mean BPI-SF component scores during Radium-223 treatment improved or were maintained from baseline, regardless of EOD or opioid use. Thus, the authors concluded that utilizing these real-world data, patients with mCRPC experienced a reduction of bone-associated pain during Radium-223 therapy, regardless of EOD or opioid use. The benefit appeared most pronounced in patients who received 5–6 cycles of Radium-223.


