(UroToday.com) As a portion of the European Society for Medical Oncology (ESMO) Annual Congress, an Educational Session focused on precision management of patients with prostate cancer was held. In this context, Dr. Jean-Emmanuel Bibault discussed the role of artificial intelligence to guide prostate cancer treatment.
Artificial intelligence uses available data to train algorithms to perform a specific task. The data used to inform these algorithms may be derived from a variety of sources including clinical data, narrative reports, imaging studies, pathology, and genomic studies. To allow for actionability, any derived algorithms must be validated on external datasets.
Artificial intelligence has, thus far, been predominately restricted to a research setting. One of the first applications in prostate cancer has been for the automation of prostate cancer diagnosis and tumor grading on prostate biopsy specimens using neural networks. This may also be applied for imaging segmentation of magnetic resonance imaging or computed tomography. This may be applied diagnostically or in the course of radiation treatment planning. In terms of surgery, this is much less well developed.
In terms of contemporary prostate cancer treatment, decisions are guided by risk stratification based predominately on clinical variables, perhaps incorporated into an algorithm and sometimes including genomic data. In the context of the ProtecT trial, Dr. Bibault emphasized that risk stratification is critical to appropriately allocate treatment to those who are likely to benefit from therapy and spare those who are not the toxicity associated with treatment.
He described an effort based on the dataset of the Prostate, Lung, Colorectal, and Ovarian (PLCO) trial examining approximately 77,000 men randomized to annual prostate cancer screening and usual care. The authors used data on prostate cancer diagnosis (including standard variables for risk stratification), medical history, physical activity, socioeconomic status, and hormonal status to predict prostate cancer mortality and all-cause mortality 10 years following a cancer diagnosis. The models for each of these outcomes performed well across a variety of metrics including accuracy, precision, recall, and others.

The authors then applied their model to distinguish patients who are at low and high risk of dying of prostate cancer with 10 years of diagnosis. As highlighted in the Kaplan Meier curve that follows, there is an early and profound splitting of these curves.
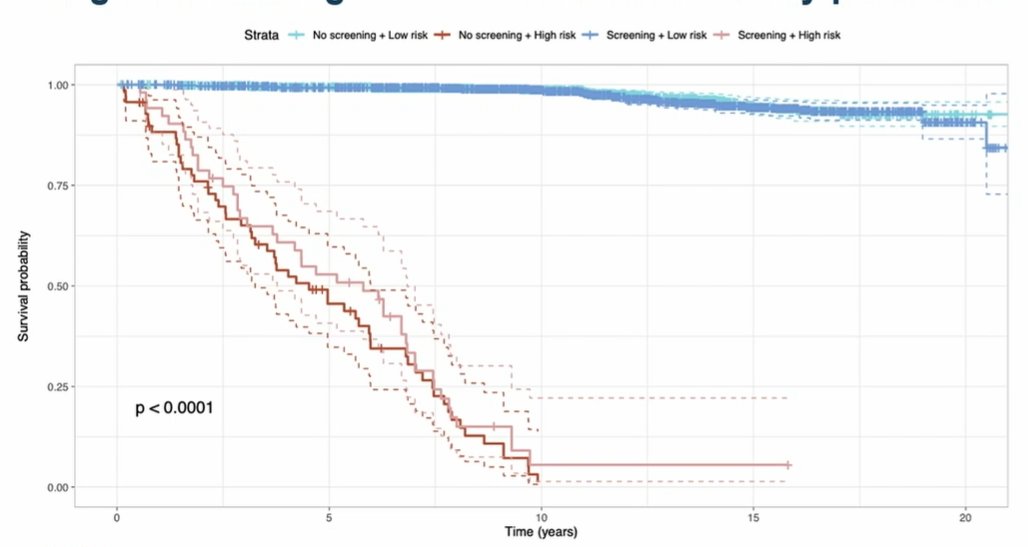
This approach significantly outperforms standard screening approaches. This approach also distinguished patients on the basis of their likelihood of dying of any cause within 10 years. Interestingly, while traditional prostate cancer characteristics (Gleason score, PSA at diagnosis, treatment approach, and T stage) influenced prostate cancer mortality, other characteristics were also influential including demographics and comorbidities.
Additionally, at the individual patient level, such an algorithm may be useful when considering cancer-related risks and competing causes of death. For patients with a low risk of prostate cancer-related death and a high risk of other cause mortality, a clinician may be inclined to avoid morbid or risky prostate cancer-related treatments. Conversely, for those with a high risk of cancer-related mortality and low other cause mortality, prostate cancer treatment is strongly warranted.
These results, while promising, require validation in prospective cohorts.


