(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 annual meeting’s prostate cancer session included a presentation by Dr. Emmanuel S. Antonarakis discussing updated results of KEYNOTE-199 with four years of follow-up assessing pembrolizumab monotherapy for docetaxel-pretreated mCRPC. KEYNOTE-199 is a multi-cohort phase II study to evaluate pembrolizumab in mCRPC with previous analyses of three cohorts of patients (Cohort 1: RECIST-measurable, PD-L1+; Cohort 2: RECIST-measurable, PD-L1−; Cohort 3: bone-predominant irrespective of PD-L1) who were previously treated with a next-generation hormonal agent and docetaxel showing durable antitumor activity and a manageable safety profile with pembrolizumab. At the 2021 ESMO meeting, Dr. Antonarakis provided an updated efficacy and safety data with 4 years of follow-up.
In KEYNOTE-199, patients received pembrolizumab 200 mg Q3W for up to 35 doses until progression, unacceptable toxicity, or withdrawal of consent. The study design is as follows:
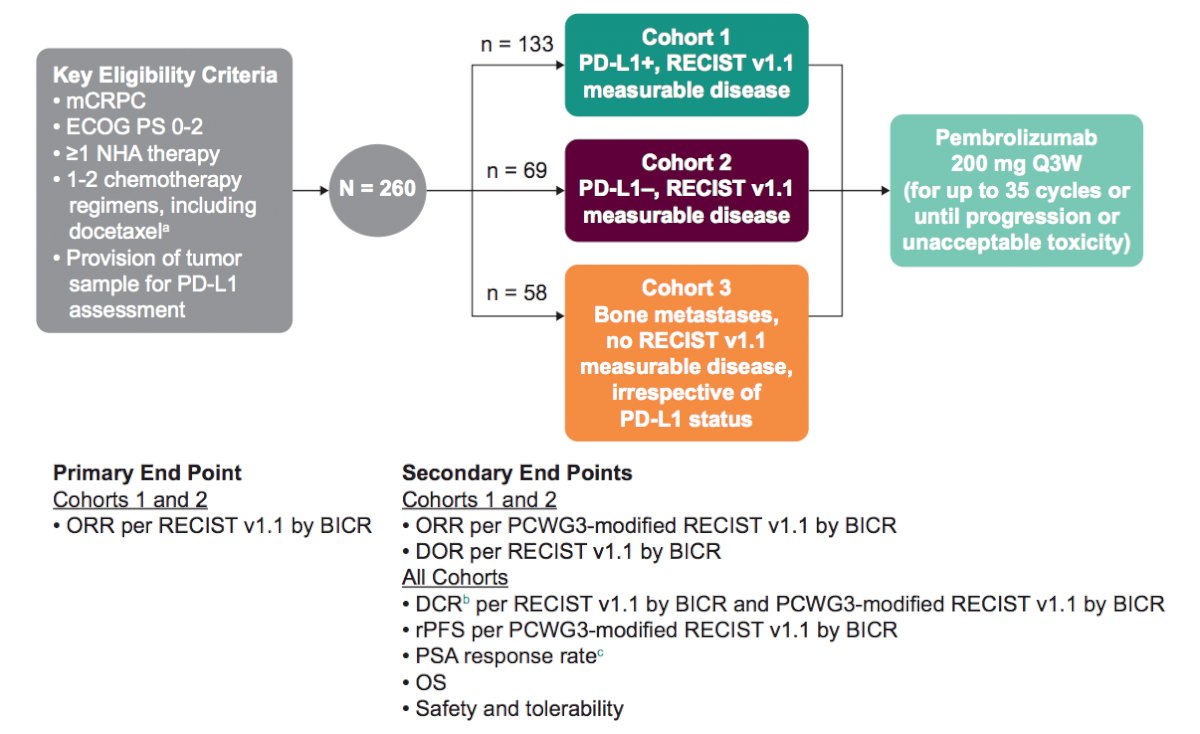
The primary endpoint was ORR (complete response + partial response) per RECIST v1.1 by blinded independent central review. Secondary endpoints were durable complete response (complete response + partial response of any duration + stable disease/non-complete response/non-progressive disease ≥6 months), duration of response, confirmed PSA response (≥50% reduction from baseline) rate, rPFS per PCWG-3-modified RECIST v1.1, OS, and safety.
Among the 258 treated patients in the trial (Cohort 1: n=133; Cohort 2: n=67; Cohort 3: n=58); 6 (2.3%) completed treatment and 252 (97.7%) discontinued treated, primarily because of progressive disease. As follows is the patient disposition stratified by cohort:

The median time from enrollment to data cutoff date was 48.1 months (range, 43.1-51.1). In patients with measurable disease, ORR was 6.0% (95% CI, 2.6-11.5; 4 complete response, 4 partial response) in Cohort 1 and 3.0% (95% CI, 0.4-10.4; 2 partial response) in Cohort 2. The median duration of response was not reached in Cohort 1 or Cohort 2, but 3 patients in Cohort 1 continued in response for ≥36 months. Additional efficacy analyses are as listed in the table:

The median overall survival for cohort 1 was 9.5 months (95% CI 6.4-11.9), for cohort 2 was 7.9 months (95% CI 5.9-10.2), and for cohort 3 was 14.1 months (95% CI 10.8-17.6):

Of all patients, treatment-related adverse events occurred in 61.2% (158/258), and grade 3-5 treatment-related adverse events occurred in 15.9% (41/258). One patient in each cohort died of a treatment-related adverse event (Cohort 1: sepsis; Cohort 2: unknown; Cohort 3: immune-related pneumonitis).
Dr. Antonarakis concluded this extended follow-up of the KEYNOTE-199 trial with the following take-home messages:
- With 4 years of follow-up data, pembrolizumab monotherapy continued to show modest antitumor activity in patients with RECIST-measurable and bone-predominant mCRPC that was previously treated with novel hormonal agents and docetaxel
- Responses to pembrolizumab monotherapy were durable in that half of patients with a confirmed response remained in response for >= 24 months, and 1 patients in cohort 1 remained in response for >=48 months
- The median OS ranged from 7.9 to 14.1 months in all cohorts
- Pembrolizumab was well tolerated and no new safety signals were observed
- The promising durability of response and durable control rate supports further exploration of pembrolizumab in combination with other agents for the treatment of mCRPC, including the KEYNOTE-641, KEYNOTE-921, and KYLYNK-010
Presented by: Emmanuel S. Antonarakis, MD, Division of Medical Oncology, Johns Hopkins School of Medicine, Sidney Kimmel Comprehensive Cancer Center, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.


