(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 annual meeting’s prostate cancer session included a presentation by Dr. Evan Yu discussing updated follow-up of the KEYNOTE-365 Cohort A trial. Data from the phase I/II KEYNOTE-365 study previously showed antitumor activity and acceptable safety of pembrolizumab + olaparib in patients with molecularly unselected, docetaxel-pretreated mCRPC enrolled in cohort A. At the ESMO 2021 congress, Dr. Yu and colleagues presented updated results for all patients after a minimum of 11.4 months of follow-up.
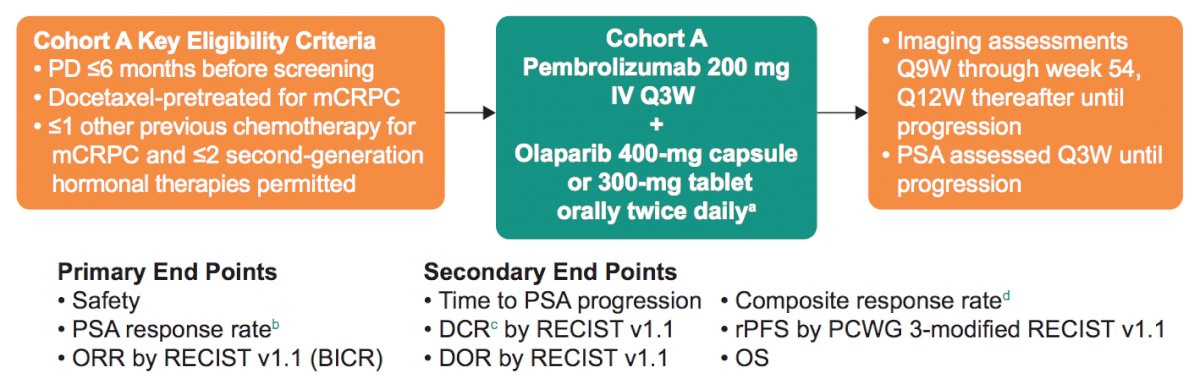
In KEYNOTE cohort A, patients with molecularly unselected, docetaxel-pretreated mCRPC whose disease progressed within 6 months before screening received pembrolizumab 200 mg IV Q3W + olaparib 400-mg capsule or 300-mg tablet orally BID. Patients could have received one chemotherapy other than docetaxel for mCRPC and ≤2 second-generation androgen receptor–targeted therapies. Primary end points were PSA response rate (decrease of ≥50% from baseline), ORR by RECIST v1.1 by blinded independent central review, and safety. Secondary end points were durable complete response by blinded independent central review (complete response or partial response of any duration + stable disease or non-complete response/non-progressive disease ≥6 months), duration of response by blinded independent central review, rPFS by PCWG3, and OS. The study design of KEYNOTE-365 Cohort A is as follows:
Among the 104 enrolled patients, 102 were treated, and 10 remained on treatment. The complete patient disposition is as follows:
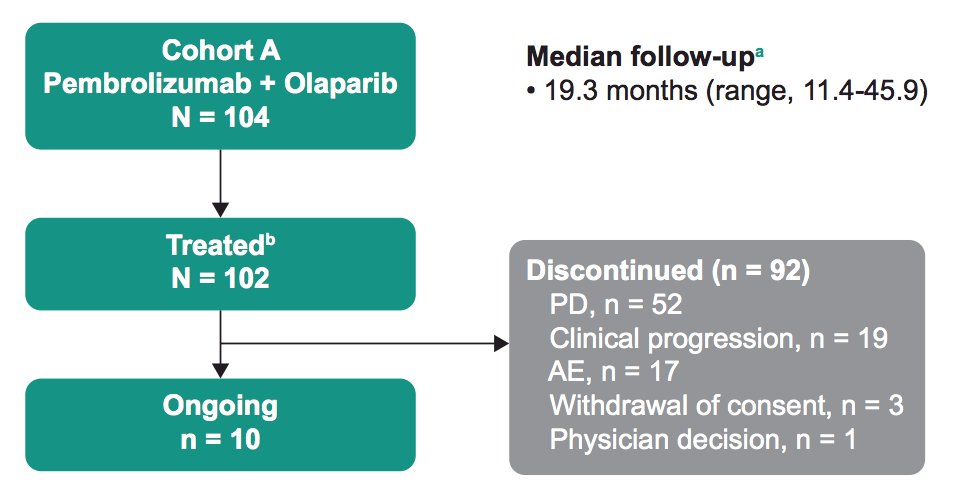
The median follow-up, defined as time from enrollment to data cutoff, was 19.3 months (range, 11.4-45.9). Confirmed PSA response rate in patients with a baseline PSA measurement (n = 102) was 14.7% (95% CI, 8.5-23.1):

In 58 patients with measurable disease, the confirmed ORR was 6.9% (95% CI, 1.9-16.7; 4 partial responses), the median duration of response was not reached (range, 7.2+ to 37.8+ months), and 2 patients had a response ≥12 months. The durable complete response rate was 26.5% (95% CI, 18.2-36.1). The median time to PSA progression was 4.0 months (95% CI 3.0-4.9), and median rPFS was 5.2 months (95% CI, 4.1-6.5), with a 12-month rate of 28.8%:

The median OS was 14.4 months (95% CI, 10.4-17.9) with a 12-month rate of 55.9%. Treatment-related adverse events occurred in 93 patients (91.2%), with the most frequent (≥30%) being anemia (41.2%), nausea (41.2%), decreased appetite (30.4%), and fatigue (30.4%). Grade 3-5 treatment-related adverse events occurred in 49 patients (48.0%), including six patients (5.9%) that died of adverse events (2 deaths were treatment related - unknown cause and myocardial infarction).
Dr. Yu concluded his presentation of the updated KEYNOTE-365 Cohort A trial with the following summary statements:
- With a minimum of 11.4 months of follow-up, pembrolizumab + olaparib continued to show modest activity in patients with molecularly unselected, docetaxel-pretreated mCRPC
- The safety and tolerability profile of pembrolizumab + olaparib combination therapy was consistent with individual profiles of each agent
- The confirmed PSA responses rate was 14.7% with an objective response rate of 6.9% and disease control rate of 26.5%
- This combination is being evaluated further in the phase III KEYLYNK-010 study (NCT03834519)
Presented by: Evan Yu, MD, Medical Oncology, University of Washington, Seattle, WA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.


