(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 virtual annual meeting’s prostate cancer session included a presentation by Dr. Nobuaki Matsubara estimating overall survival with subsequent treatment effect by applying inverse probability of censoring weighting in the LATITUDE study.1
In LATITUDE, abiraterone acetate plus prednisone added to androgen deprivation therapy (ADT) demonstrated significant overall survival (OS) benefit in patients with high-risk metastatic castration-sensitive prostate cancer (mCSPC), compared with placebo. Around 57% of patients received life-extending subsequent therapies after placebo discontinuation. In oncological clinical studies, sensitivity analyses for OS are usually conducted to estimate the true treatment effect, however there is no report to adjust for patients who did not receive subsequent therapies. It is controversial whether survival benefits would remain if all patients in the placebo group received life-extending subsequent therapies. Presented at the ESMO 2021 virtual congress, this is the first analysis to estimate the real treatment effect adjusting for patients without life-extending subsequent therapies.
For this study, Dr. Matsubara and colleagues conducted a post-hoc analysis of data from the LATITUDE study, applying intention-to-treat analysis and a naïve censoring and inverse probability of censoring weighting (IPCW) methods to the placebo group to reveal the proportion of life-extending subsequent therapies in patients initially treated with placebo. In a naïve-censoring approach, patients who did not receive life-extending subsequent therapies were censored at the date of protocol treatment discontinuation. The IPCW method in this study could represent conditions in which all patients had switched to life-extending subsequent therapies, unless they died possibly related to protocol treatment, adjusted by baseline and time-varying covariates. The OS hazard ratio of abiraterone acetate plus prednisone versus placebo and associated 95% confidence interval were estimated using a Cox proportional hazards model.
Among 581 patients in the placebo group eligible for life-extending subsequent therapies, 344 (59.2%) patients received life-extending subsequent therapies. As follows is a schematic of the study design:
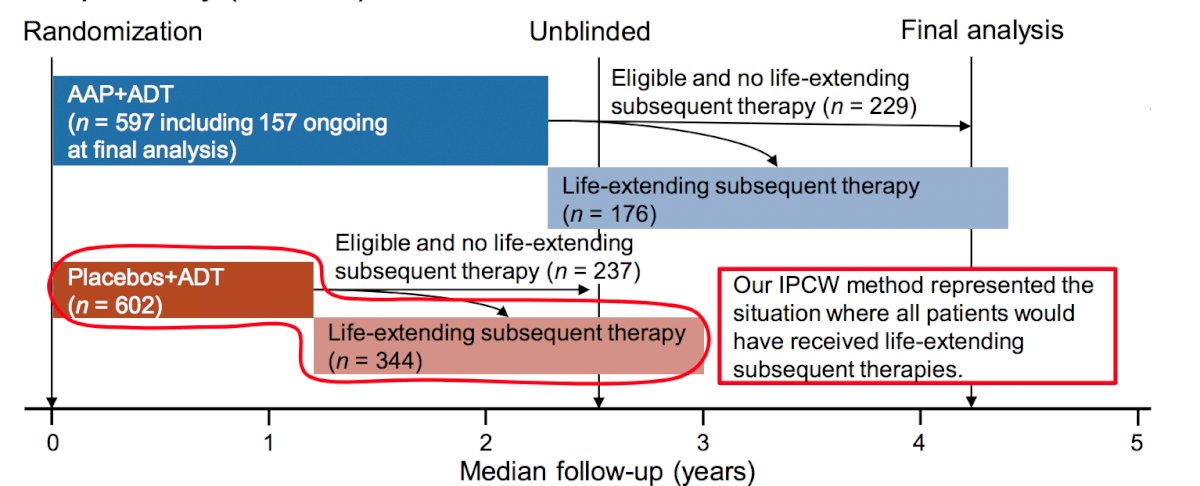
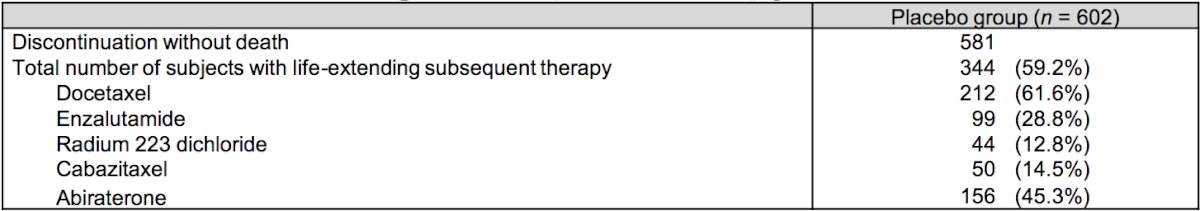
In those who discontinued study treatment, patients with ECOG performance status 2 and those from Eastern Europe had a greater proportion that did not receive life-extending subsequent therapy. Alternatively, the opposite tendency was observed in those with Gleason Score >8 and from Western Europe in the placebo group. The median study treatment duration in the placebo group was 15.1 months (range 0.9-51.3) in patients with life-extending subsequent therapy, and 11.6 (range 0.7-49.7) months in those without. As follows is a table summarizing the life-extending subsequent therapies among patients in the placebo group:
The naïve censoring analysis and the IPCW method showed a trend toward a prolongation of OS in the placebo group:

The hazard ratio for OS with abiraterone acetate plus prednisone in the IPCW analysis was higher than that in the unadjusted intention-to-treat analysis (HR 0.732 versus 0.661) and lower than that in the naïve censoring analysis (HR 0.732 versus 0.788):

Dr. Matsubara concluded this presentation with the following take home points:
- Adding abiraterone acetate plus prednisone to ADT in patients with high-risk mCSPC reduced the risk of death by 26.8% and provided 14.7 months of additional survival if all patients in the placebo group received life-extending subsequent therapies
- This post hoc analysis confirmed the importance of earlier intensive treatment for patients with high-risk mCSPC regardless of receiving life-extending subsequent therapy
- The IPCW method was useful for estimating OS with subsequent treatment effects in clinical studies where a small population receives subsequent therapy, given that the question of OS and subsequent therapy posed by the LATITUDE study is commonly encountered in other clinical trials
Presented by: Nobuaki Matsubara, Department of Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:


