(UroToday.com) In the Proffered Paper Session of the European Society for Medical Oncology (ESMO) Annual Congress focusing on prostate cancer, Dr. Shahneen Sandu presented the preliminary results based on an interim analysis of the PRINCE trial assessing the role of 177-Lu-PSMA-617 in combination with pembrolizumab in men with metastatic castration-resistant prostate cancer (mCRPC).
Dr. Sandu began with background explaining that 177Lu-PSMA-617 (Lu-PSMA-617) is a radiolabelled small-molecule that delivers β radiation to tumors expressing PSMA. Based on both the TheraP and VISION studies, there is evidence that Lu-PSMA-617 has meaningful efficacy in mCRPC. However, recurrence and progression of the disease is common and therefore, there is an ongoing need for further treatment options. While pembrolizumab, an anti-programmed death 1 inhibitor, has modest single-agent anti-tumour activity in mCRPC, the authors hypothesised that β radiation can induce tumour cell death potentially releasing tumour-associated antigens that would be potentially immunogenic. Thus, the addition of pembrolizumab to Lu-PSMA-617 may improve responses.
In the PRINCE trial, patients with mCRPC patients with high PSMA expression without discordant FDG avid disease on paired PSMA and FDG PET/CT scans received up to 6 cycles of Lu-PSMA-617 (6-8 GBq) every 6 weeks in conjunction with 200mg of pembrolizumab every 3 weeks for up to 2 years. Patients could have received prior docetaxel and were required to have had prior novel androgen targeting with enzalutamide, abiraterone, or apalutamide.

The co-primary endpoints were the safety of the treatment regime and 50% PSA response rate (PSA50-RR) while secondary endpoints included PSA-progression-free survival (PSA-PFS), radiographic PFS (rPFS), and overall survival (OS).
The authors have, to date, enrolled 37 patients with a median age of 72 years. In terms of prior therapy, 73% had previously received docetaxel 73% and all had received prior androgen receptor-targeted agents. In terms of study treatment, patients received a median of 4 cycles of Lu-PSMA-617 and 8 doses of pembrolizumab.
Common treatment-related adverse events (TRAE) (≥10%) were predominately low grade (1-2) and included xerostomia (76%), fatigue (43%), diarrhoea (11%), rash (22%), nausea (24%), elevated ALT (11%), pruritus (19%), and bone pain (11%). Haematologic TRAE included G2-3 anemia (8%), G1-2 thrombocytopenia (14%), and G1 neutropenia (3%). In terms of grade 3 immune-related AEs, one patient each had mucosal pemphigus, myasthenia gravis, optic neuritis, pancreatitis, pneumonitis, nephritis, amylase elevation, and type 1 diabetes, while 2 (5%) patients had colitis. Four (11%) patients discontinued treatment due to toxicity, all as a result of pembrolizumab.

With a median follow-up of 38 weeks, the PSA50-RR was 73% (27/37 [95% CI: 56-86]).
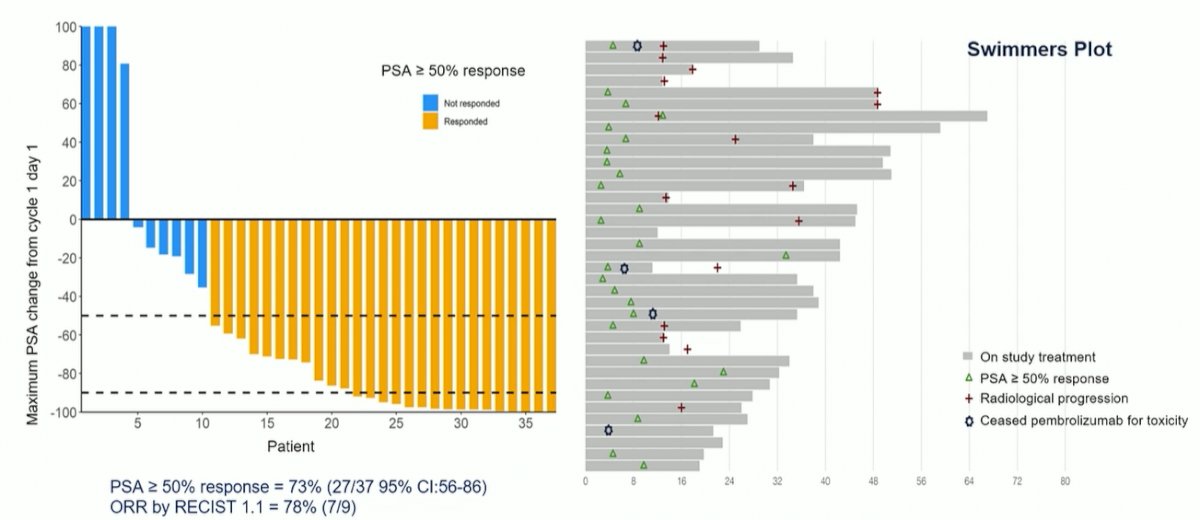
Notably, approximately 10% of patients clearly have primary resistance to this treatment approach, as seen to the left of the waterfall plot.
Among the 9 patients with RECIST measurable disease, 7 (78%) had a partial response. Further, rPFS and PSA-PFS at 24 weeks were 64% (95% CI: 45-79) and 68% (95% CI: 50-81) respectively.

The authors further assessed PSMA expression on CTCs from baseline to week 12 among 29 patients, finding that 61% of patients (11/18) cleared from PSMA+ cells to not detectable while 83% had decreased (15/18) and all patients without evidence of PSMA+ CTCs at baseline (11/11) maintained this.
She further contrasted the PRINCE study with other work assessing the combination of Lu-PSMA-617 and pembrolizumab. These two studies utilized differing treatment approaches, with PRINCE employing a much greater utilization of Lu-PSMA-617, and found differing results, with PRINCE demonstrating much higher rates of both PSA response and RECIST 1.1 response. Further, the PRINCE trial used PSMA expression as a key inclusion criterion and FDG selection was used for exclusion as well.

Dr. Sandhu, therefore, concluded that the combination of Lu-PSMA-617 and pembrolizumab has promising activity and manageable toxicity which was consistent with those of single-agent Lu-PSMA-617 and pembrolizumab.
Related Content: ESMO 2021: Discussion on the PRINCE Trial of 177Lu-PSMA-617 in Combination With Pembrolizumab for Metastatic Castration-Resistant Prostate Cancer


