(UroToday.com) In the Presidential Symposium 2 of the European Society for Medical Oncology (ESMO) Annual Congress focusing on prostate cancer, Dr. Daniel Petrylak presented results of the CheckMate 9KD cohort A2 final analysis assessing nivolumab + rucaparib for men with chemotherapy (CT)-naïve metastatic castration-resistant prostate cancer (mCRPC).
Dr. Petrylak emphasized that CheckMate 9KD combined nivolumab, an anti-PD-1 agent, with rucaparib, docetaxel, or enzalutamide for mCRPC. In cohort A2 specifically, patients were eligible if they had not previously received either chemotherapy or a PARP inhibitor for mCRPC. They, however, had to have received 1 or two lines of novel hormonal therapy with prior abiraterone and/or enzalutamide for mCRPC.
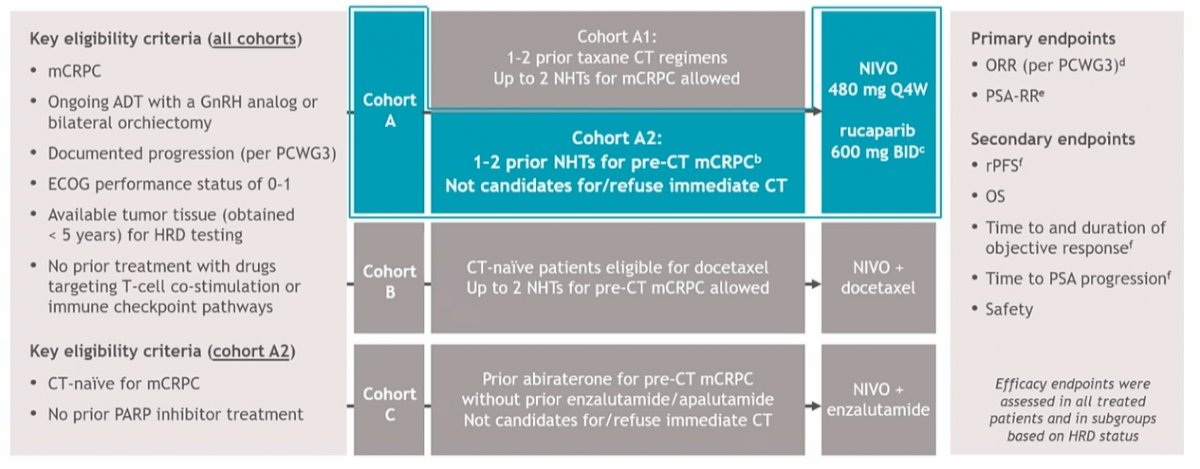
Following enrollment, patients received nivolumab 480 mg Q4W + rucaparib 600 mg BID until disease progression/unacceptable toxicity (nivolumab dosing ≤ 2 y). The coprimary endpoints were objective response rate (ORR) per PCWG3 criteria and prostate-specific antigen response rate (PSA-RR) in all treated patients (biomarker unselected) and in subgroups defined by homologous recombination deficiency positive (HRD+) tumors. Notably, HRD status was determined before enrollment. Secondary endpoints included radiographic progression-free survival (rPFS), overall survival (OS), and safety.
The authors included 71 patients who had a median age of 73 years (range, 51–87 y). In terms of disease burden, 23.9% had visceral metastases and 54.9% had measurable disease. The median treatment duration was 4.6 months for nivolumab and 5.5 months for rucaparib. The median follow-up was 17.5 months. At the time of database lock on March 12, 2021, 65 (92%) of patients had discontinued treatment, primarily for disease progression.
In the overall cohort, the confirmed ORR was 15.4% and the confirmed PSA response rate was 27.3%. However, among patients with HRD+ tumors, confirmed ORR was 25.0%, and confirmed PSA response was 41.9%. These rates were even higher (33.3% and 84.6%, respectively) among patients with mutations in BRCA1 or BRCA2.

In the overall cohort, median rPFS was 8.1 months (95% CI 5.6-10.9) though this again was much higher among those with HRD+ tumors (10.9 months, 95% CI 6.7-12.0). The median overall survival was 20.2 months (95% CI 14.1-22.8) in the overall population and 22.7 months (95% CI 14.1-NR) among those with HRD+ tumors. However, the authors did not perform formal statistical comparisons between these groups.
Any-grade treatment-related AEs (TRAEs) occurred in 90.1% of patients, most commonly nausea (40.8%) and anemia (32.4%). Grade ≥ 3 TRAEs occurred in 50.7% of patients, most commonly anemia (14.1%) and increased ALT (12.7%). TRAEs led to discontinuation in 23.9% of patients while no treatment-related deaths were reported.

Dr. Petrylak thus concluded that nivolumab + rucaparib is active in patients with HRD+ chemotherapy-naïve mCRPC, particularly among those with BRCA mutations. Longer follow-up is needed to better characterize the clinical benefits of adding nivolumab to rucaparib for this population.
Presented by: Daniel P. Petrylak, MD, Yale School of Medicine, New Haven, CT, United States of America


