(UroToday.com) The European Society of Medical Oncology (ESMO) 2021 annual meeting’s non-prostate cancer proffered paper session included a presentation by Dr. Alexandros Papachristofilou discussing results from the SAKK 01/10 phase II trial testing a single dose of carboplatin followed by involved-node radiotherapy as curative treatment for seminoma stage IIA/B. Seminoma stage IIA/IIB is defined as one or more enlarged lymph nodes in the retroperitoneum or pelvis that are either <= 2 cm (IIA) or 2-5 cm (IIB). Standard treatment options for patients with seminoma clinical stage IIA/B are either extensive “dog-leg” para-aortic/pelvic radiotherapy or 3-4 cycles of cisplatin-based combination chemotherapy with a 3-year progression free survival (PFS) of ~90% (89% with radiotherapy; 92% with chemotherapy), but potential acute and late toxicities. As follows is a table summarizing toxicity post-radiotherapy and post-chemotherapy:

The pattern of relapse after chemotherapy is typically the retroperitoneum, whereas the pattern of relapse for radiotherapy is mainly the supradiaphragmal lymph nodes. SAKK 01/10 is a trial of the Swiss Group for Clinical Cancer Research (SAKK) and the German Testicular Cancer Study Group (GTCSG) aiming at reducing therapy toxicity while preserving efficacy in clinical stage IIA/B seminoma by combining deescalated chemotherapy and radiotherapy.
SAKK 01/10 is a multicenter, single arm, phase II study in patients with clinical stage IIA/B seminoma (de novo or relapse on active surveillance). Treatment consisted of 1 cycle carboplatin AUC7 followed by involved-node radiotherapy (IIA: 30 Gy; IIB: 36 Gy):

The primary endpoint is 3-year PFS. With a target 3-year PFS of 95%, 120 patients were required to show that the lower limit of a two-sided 90% confidence interval is >90%. Secondary endpoints include time to progression (TTP), overall survival, patterns of tumor progression, acute and chronic adverse events, including secondary malignancies.
A total of 120 patients were included from October 2012 until June 2018 in 20 centers in Switzerland and Germany. There were 116 patients eligible and started on treatment per protocol (IIA: 46, IIB: 70; de-novo: 76, relapsing: 40), with a median age of 40 years (range 22-68), and median number of metastatic lymph nodes of 2 (range: 1-8). Minimum follow up from inclusion of last patient is 3 years, with a median follow-up time of 4.5 years (range: 0.8 years - 8.1 years). The median carboplatin dose was 987 mg (range: 560-1920), with a dose deviation in 24% of patients, and the median planning target volume for radiotherapy was 294.0 cm3 (range: 24-1047). Importantly, the median planned target volume was ~25% of a typical, standard of care dog-leg planned target volume, thus a 75% median decrease in irradiated volume.
The 3-year PFS rate is 93.7% (90% CI 88.5%, 96.6%), with a 3-year PFS rate of 95.2% (90% CI 85.5%, 98.5%) for IIA patients and 92.6% (90% CI) for IIB patients:
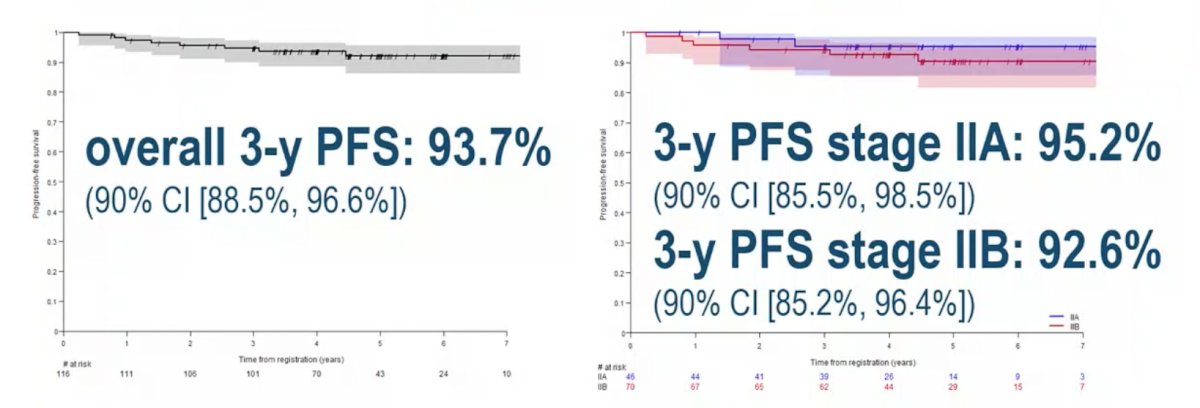
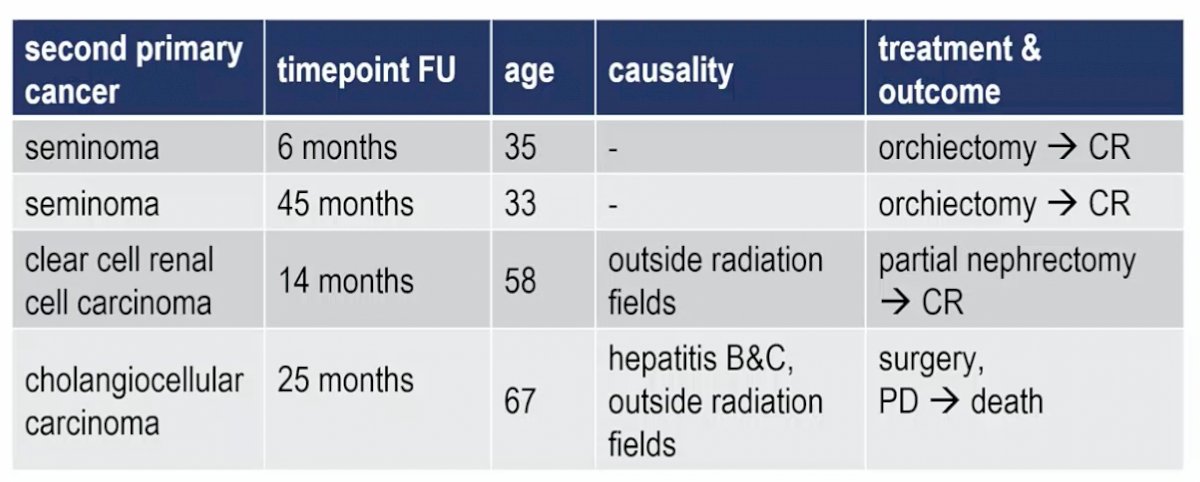
In total, 7 patients developed a recurrence (1 clinical stage IIA, 6 clinical stage IIB), all outside the radiotherapy volumes and all salvaged with conventional chemotherapy, with a median time to relapse of 16.7 months and a 3-year relapse-free rate of 94.6% (90% CI 89.6-97.2%). All relapses outside the planned target volume were salvaged with conventional chemotherapy (BEP/TIP). The most common grade 3/4 adverse events during treatment were thrombocytopenia (3.2%) and neutropenia (2.5%), with no treatment-related late adverse events. There were four secondary primary cancers, including two seminomas, one clear cell renal cell carcinoma, and one cholangiocarcinoma:
Dr. Papachristofilou concluded his presentation of the SAKK 01/10 trial with the following summary notes:
- SAKK 01/10 is the largest completed prospective trial in seminoma stage IIA/B to date
- A favorable 3-year PFS using the combination of single dose carboplatin AUC7 and 30-36 Gy involved node radiotherapy was achieved
- Toxicity is so far very low and certainly considerably less than with standard chemotherapy or radiotherapy
- Based on the data, this treatment regimen can be viewed as an attractive option in clinical stage IIA/B seminoma
- Long term toxicity, especially secondary malignancies, requires long-term follow-up
- Long-term data from Hodgkin’s lymphoma demonstrate that de-escalated combined modality treatment (chemotherapy + radiotherapy) dose not lead to more secondary malignancies than intensive single-modality treatment
Presented by: Alexandros Papachristofilou, MD, Department of Radiation Oncology, University Hospital of Basel, Basel, Switzerland
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.


