(UroToday.com) The 2022 ESMO annual meeting featured a urothelial carcinoma session, including a presentation by Dr. Enrique Pulido discussing outcomes of PD-L1 expression on immune cells by SP142 in patients treated with atezolizumab for untreated metastatic urothelial cancer. PD-L1 assays use various methods (e.g. scoring tumour and/or immune cells) to predict PD-L1/PD-1 blockade outcomes. Data suggest that the clinically relevant PD-L1–expressing immune cells are dendritic cells.1 At the 2022 ESMO meeting, Dr. Pulido and colleagues probed the clinical and immunologic basis underlying the trend toward favorable OS with atezolizumab alone (anti–PD-L1; Arm B) vs placebo + platinum/gemcitabine chemotherapy (Arm C) in patients with high PD-L1 by SP142 in the Phase 3 IMvigor130 trial (ITT: HR 0.68, 95% CI: 0.43 to 1.08; cisplatin ineligible: HR 0.53, 95% CI: 0.30 to 0.94).2 They hypothesized that the SP142 and 22C3 assays may be differentially associated with OS in IMvigor130, which may be related to PD-L1 localization on specific immune cells such as dendritic cells.
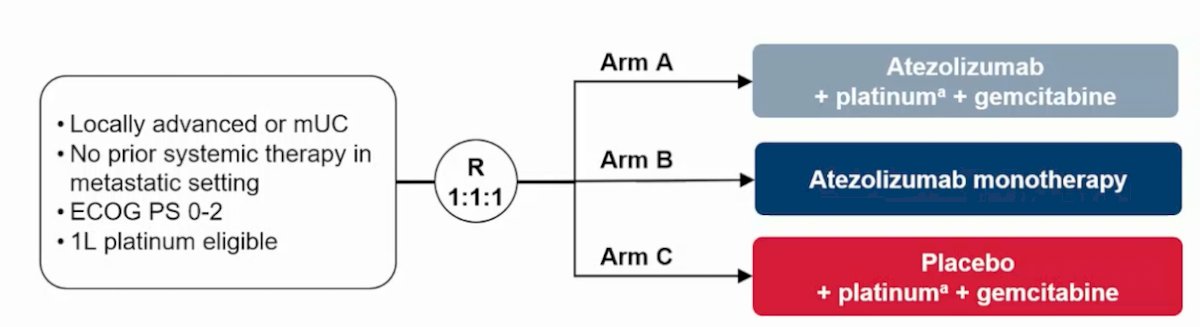
The trial schema for IMvigor130 randomizing patients to atezolizumab + platinum + gemcitabine (Arm A) vs atezolizumab monotherapy (Arm B) vs placebo + platinum + gemcitabine (Arm C) is as follows:
This post hoc analysis studied associations between two PD-L1 immunohistochemistry tests (VENTANA SP142 and Dako 22C3) and OS in patients with archival tumors (biomarker-evaluable patients) in IMvigor130 Arms B and C (cutoff June 14, 2020). Assay cutoffs were SP142 IC ≥5% (IC2/3)/<5% (IC0/1) and 22C3 combined positive score (CPS) ≥10/<10. Samples were also stained for immune cells subtypes.
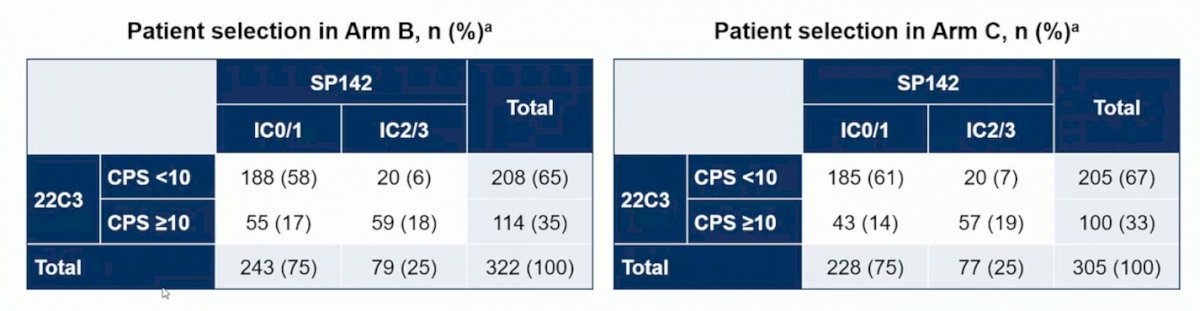
Demographics and OS of biomarker-evaluable patients (n = 627) and ITT (n = 719) patients were similar. The SP142 and 22C3 assays were found to select for different sets of patients:
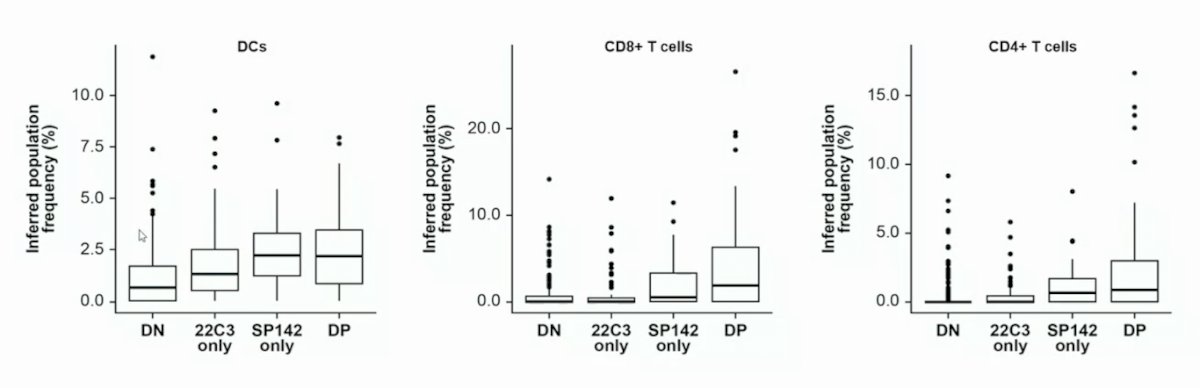
In deconvoluting the cellular components associated with PD-L1 staining, the investigators found that SP142 preferentially co-localized with dendritic cells (DC-LAMP, a DC-specific marker) as opposed to other myeloid subsets. Bulk RNA sequencing deconvolution suggested that a higher frequency of dendritic cells and T cells occurred in SP142 IC2/3 than in IC0/1 tumors:
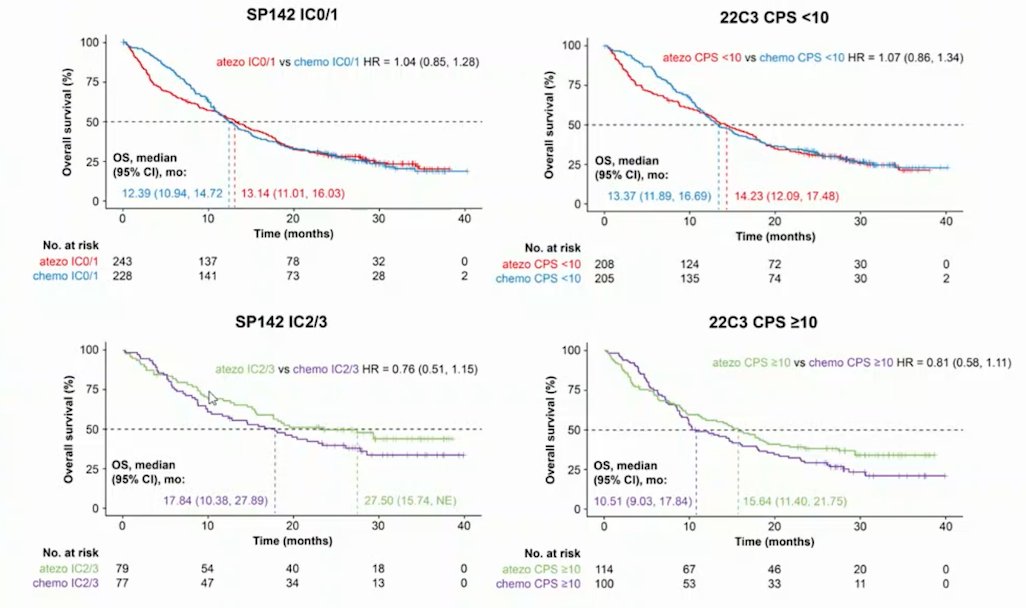
SP142 scoring appeared to have better prognostic ability than 22C3 CPS scoring in patients receiving atezolizumab monotherapy:
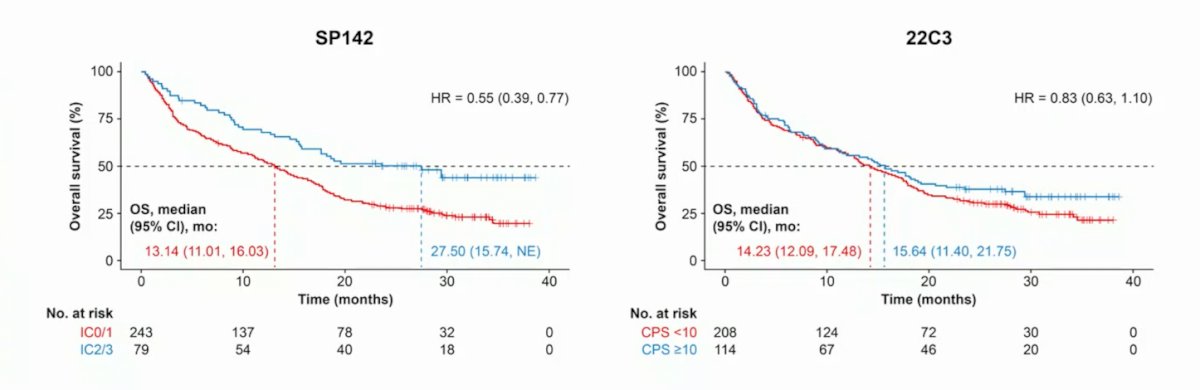
Additionally, SP142 PD-L1 expression was associated with better prognosis and was a predictive biomarker for atezolizumab monotherapy versus chemotherapy. The 22C3 assay selected a larger pool of patients receiving atezolizumab monotherapy with high PD-L1 expression (CPS >= 10; 35%) than the SP142 assay (IC2/3; 25%) thus diluting the PD-L1 high population. Patients in Arms B and C who were selected by the 22C3 assay had shorter median OS than those selected by the SP142 assay:
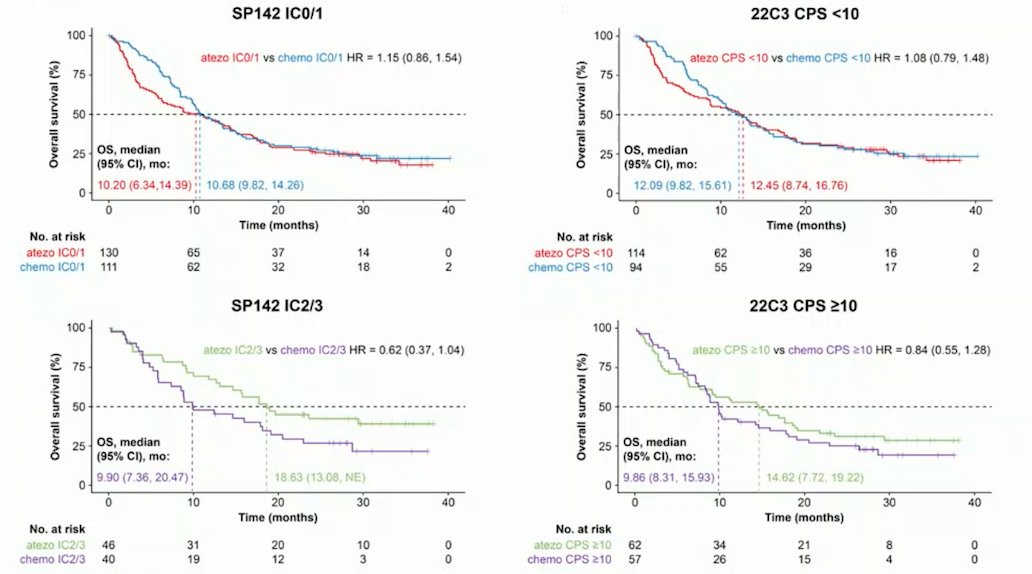
Similarly, high SP142 PD-L1 expression was associated with better prognosis and was a predictive biomarker for atezolizumab monotherapy versus chemotherapy in patients who were cisplatin ineligible:
Finally, longer OS was associated with SP142 IC2/3 + 22C3 CPS >=10 tumor status, and shorter OS was observed in patients with tumors staining for SP142 IC0/1 + 22C3 CPS >= 10:

Dr. Pulido concluded his presentation by discussing outcomes of PD-L1 expression on immune cells by SP142 in patients treated with atezolizumab with untreated metastatic urothelial cancer with the following take-home points:
- This IMvigor130 exploratory analysis showed that SP142 PD-L1 staining preferentially co-localized with dendritic cells compared with other myeloid subsets
- In the biomarker-evaluable population, the SP142 assay was found to be both prognostic and predictive, whereby the SP142 assay was associated with OS while the 22C3 assay was not
- Combined analysis with the SP142 and 22C3 assays showed longer OS with tumors staining for high PD-L1 expression by both SP142 and 22C3 assays and shortest OS with tumors staining for high PD-L1 by the 22C3 but not the SP142 assays
- PD-L1-expressing dendritic cells may underlie the longer OS with atezolizumab in SP142 IC2/3 vs IC0/1 tumors in patients with metastatic urothelial carcinoma
- These findings reinforce preclinical data, demonstrating the particular importance of PD-L1-expressing dendritic cells
Presented by: Enrique G. Pulido, Medical Oncology Department, MD Anderson Cancer Center Madrid, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- Oh SA, Wu DC, Cheung J, et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer. 2020 Jul;1(7):681-691.
- Galsky MD, Arranz Arija JA, Bamias A, et al. Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomized, placebo-controlled phase 3 trial. Lancet. 2020 May 16;395(10236):1547-1557.


