(UroToday.com) The 2022 European Society of Medical Oncology (ESMO) annual meeting featured a urothelial carcinoma session, including a presentation by Dr. Tom Powles discussing genomic biomarkers in peripheral blood from patients enrolled in the JAVELIN Bladder 100 trial of avelumab first-line maintenance in advanced urothelial carcinoma.
The relationship between blood biomarkers, tumor immunity, and benefit from immune checkpoint inhibitors is not well understood. The JAVELIN Bladder 100 trial (NCT02603432) demonstrated that avelumab first-line maintenance prolongs overall survival (OS) in patients with advanced urothelial carcinoma that has not progressed with first-line chemotherapy.1 Additionally, retrospective tumor biomarker analyses indicated that tumor mutation burden and tumor immune activity are associated with OS benefit from maintenance avelumab.2 As such, host factors have been reported to influence the efficacy of immune-checkpoint inhibitors through interactions in the tumor microenvironment:

Furthermore, analysis of chromatin conformation has the potential to identify host immune factors in circulating white blood cells that are associated with anti-tumor immune activity. DNA packaging into chromatin plays a crucial role in regulating gene expression in response to developmental and environmental cues, and gene loci can assume distinct chromatin structures (ie. loops) associated with differential gene activity. Blood biomarkers associated with tumor immune gene signatures in JAVELIN Bladder 100 may provide mechanistic insight into the development of antitumor immunity and indicate patient subpopulations that could benefit from immune checkpoint inhibitors. Dr. Powles and colleagues used EpiSwitch analysis of peripheral blood to identify structural-functional epigenetic changes in genomic architecture, designated chromatin conformation signatures.
Peripheral blood from 496 patients in the JAVELIN Bladder 100 trial underwent EpiSwitch analysis by PCR assay of 3D genomic templates prepared from fixed intact nuclei:

Tumor immune activity was scored using a gene expression signature (JAVELIN Renal 101 Immune). Chromatin conformation signatures association with tumor JAVELIN Renal 101 Immune scores was determined via univariate and multivariate analyses of a training set of 80 specimens.
This study identified 25 chromatin conformation signatures from the training set. One chromatin conformation signature was proximal to POU2F2, a transcription factor instrumental in B cell development. Patients whose peripheral blood lacked the POU2F2 chromatin conformation signatures had elevated tumor JAVELIN Renal 101 Immune scores, corresponding to increased gene expression associated with tertiary lymphoid structures:

The POU2F2 chromatin conformation signatures may therefore influence key steps in tertiary lymphoid structures formation, and POU2F2 expression in tumors may be associated with maintenance avelumab survival benefit:
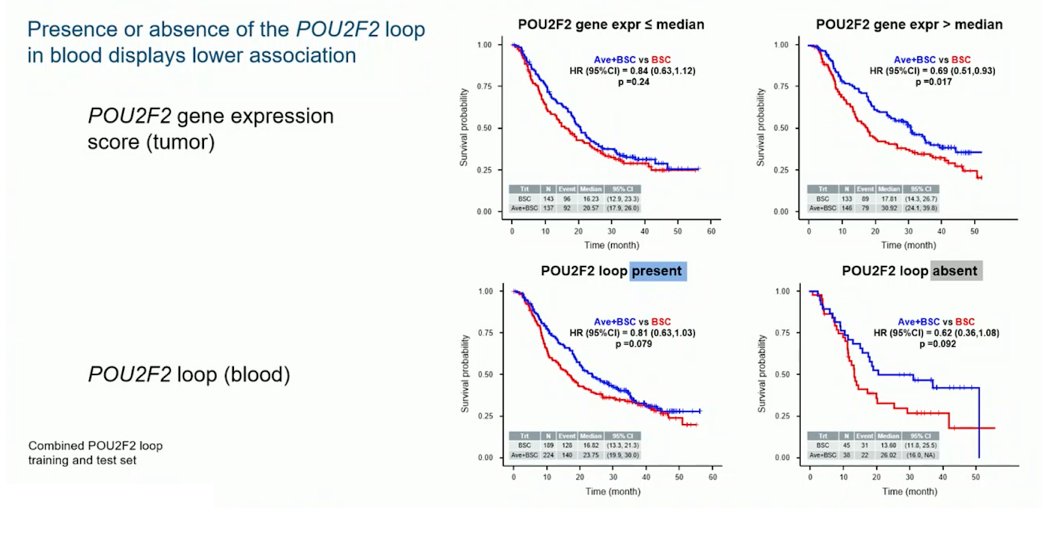
Furthermore, absence of the POU2F2 loop in blood may identify a group of tumor mutation burden-low patients with improved maintenance avelumab survival benefit:

Thus, chromatin conformation signatures affecting the ability to form tertiary lymphoid structures may influence response to avelumab at low tumor mutation burden. Dr. Powles notes that there are several limitations associated with this study, including (i) the JAVELIN Bladder 100 peripheral blood specimen set representing a single time point in the treatment cycle, and applicability to other time points should be investigated, (ii) these analyses were exploratory in nature and not corrected for multiple testing, therefore further statistical validation is required, and (iii) bioanalytical validation of the POU2F2 loop is also needed to confirm fitness for clinical applications.
Dr. Powles concluded his presentation discussing genomic biomarkers in peripheral blood from patients enrolled in the JAVELIN Bladder 100 trial of avelumab first-line maintenance in advanced urothelial carcinoma with the following take-home messages:
- Tertiary lymphoid structures appear relevant for predicting outcomes with maintenance avelumab
- Chromatin conformation loops measured in white blood cell nuclei were associated with the JAV-immuno signature, with the strongest associated with the POU2F2 loop
- The presence of the POU2F2 loop was associated with reduced POU2F2 expression and function, as well as tertiary lymphoid structure biomarkers, suggesting a negative regulatory role
- Ongoing research is assessing the potential use of chromatin loops identified by the EpiSwitch platform to form chromatin conformation signatures that may enrich patients that benefit from maintenance avelumab
- The predisposition to form tertiary lymphoid structures in response to inflammatory stimuli may be a host factor influencing response to immune checkpoint inhibitors
- Measurement of circulating chromatin loops may be a useful method for evaluating systemic versus tumor-specific biomarkers

Presented by: Thomas B. Powles, MBBS, MRCP, MD, Department of Genitourinary Oncology, Barts Cancer Institute, Experimental Cancer Medicine Centre, Queen Mary University of London, St Bartholomew’s Hospital, London, UK
Co-Authors: S. Sridhar2, J. Bellmunt3, C.N. Sternberg4, P. Grivas5, E. Hunter6, M. Dezfouli7, M. Salter8, R. Powell9, A. Dring10, J. Green9, A. Akoulitchev11, R. Amezquita12, K. Ching13, J. Pu14, S. Deng15, A. di Pietro16, C.B. Davis17
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Powles T, Sridhar SS, Loriot Y, et al. Avelumab maintenance in advanced urothelial carcinoma: Biomarker analysis of the phase 3 JAVELIN Bladder 100 trial. Nat Med. 2021 Dec;27(12):2200-2211.


