(UroToday.com) In the Urothelial Cancer poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Koshkin presented the rationale and design of a novel trial in progress of Disitamab vedotin (DV; RC48-ADC) is an investigational antibody–drug conjugate (ADC) comprised of a HER2-directed monoclonal antibody conjugated to monomethyl auristatin E (MMAE) via a protease-cleavable linker.
While there are a number of therapies available including cytotoxic chemotherapy and immunotherapy, 5-year survival for patients with metastatic urothelial carcinoma (UC) remain very low. Nearly half of all patients are not eligible or cannot tolerate standard cisplatin-based first line (1L) chemotherapy Thus, opportunity still exists to improve outcomes in both 1L and later lines of therapy. Recently, antibody drug conjugates (ADC) have been developed and gained use in UC.
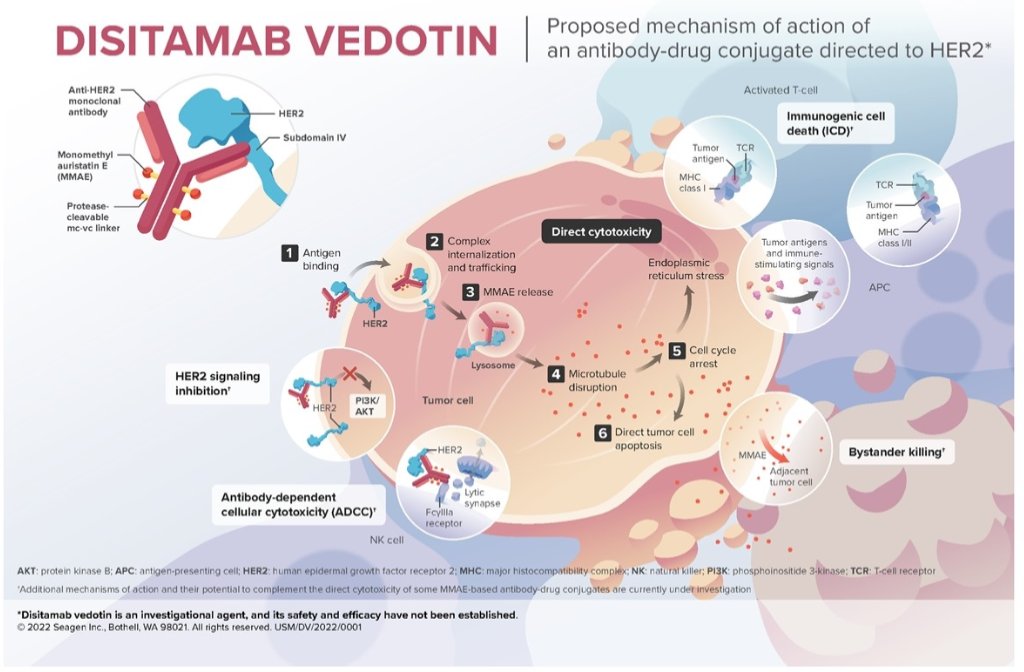
Human epidermal growth factor receptor 2 (HER2) is overexpressed in multiple malignant tumors and overexpression of HER2 may be prognostic in advanced UC, with poorer outcomes. To date, there are no HER2-directed therapies currently approved for locally advanced unresectable or metastatic UC (LA/mUC). Disitamab vedotin (DV; RC48-ADC) is an investigational antibody–drug conjugate (ADC) comprised of a HER2-directed monoclonal antibody conjugated to monomethyl auristatin E (MMAE) via a protease-cleavable linker. DV elicits antitumor activity through MMAE-directed cytotoxicity and bystander effect. DV is conditionally approved in LA/mUC and gastric cancer in China and was granted Breakthrough Therapy designation by the FDA for second-line treatment of HER2-expressing LA/mUC after platinum-containing chemotherapy.
The RC48G001 trial (NCT04879329) is a is a phase 2, multicohort, open-label, multicenter trial to evaluate DV in patients with HER2-expressing LA/mUC. There are two 2 cohorts included in the study defined by HER2 expression (by immunohistochemistry [IHC]) and gene amplification (by in situ hybridization [ISH]) by central laboratory: Cohort A (IHC 3+, or 2+ and ISH+) and Cohort B (IHC 2+ and ISH-, or IHC 1+). There is an additional third cohort (Cohort C) which may include patients with either positive or negative HER2 expression.

To be eligible, patients must have measurable disease per RECIST v1.1, ECOG Performance Status of 0 or 1, and must have received 1 or 2 lines of prior systemic therapy, including 1 line of platinum-containing chemotherapy. The primary endpoint is confirmed objective response rate (ORR) assessed by blinded independent central review. Secondary endpoints include ORR assessed by investigator, duration of response, progression-free survival, disease control rate, overall survival, safety, and pharmacokinetic parameters. Enrollment for cohorts A and B is ongoing in North America and the European Union and is planned in other parts of the world.
Presented by: Vadim S. Koshkin, MD, University of California San Francisco, San Francisco, CA
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


