Eligible patients in JAVELIN Bladder 100 had unresectable locally advanced or metastatic urothelial carcinoma that had not progressed with first-line platinum-based chemotherapy. Patients were randomized 1:1 to the Avelumab or control arm following an interval of 4-10 weeks from the end of first-line chemotherapy. The trial schema for JAVELIN Bladder 100 is as follows:
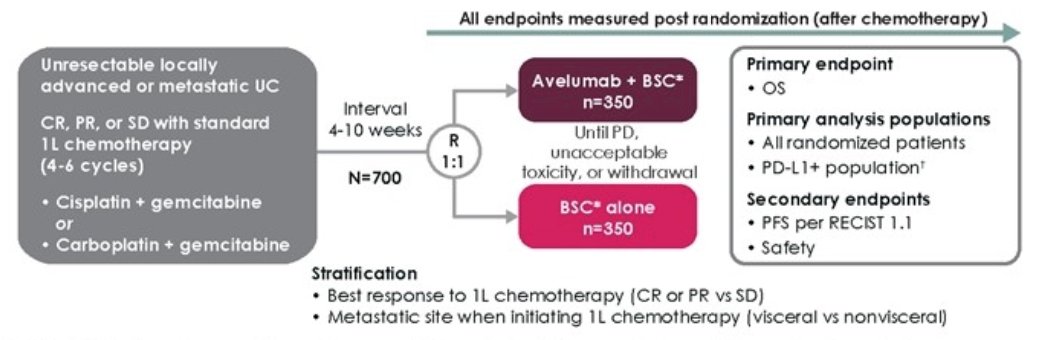
Study treatment was continued until confirmed progression, unacceptable toxicity, or withdrawal of consent.
After a median follow-up of 38.0 months in the Avelumab arm (data cutoff June 4, 2021; ≥2 years in all patients), 118/350 patients (33.7%) had received ≥12 months of treatment. Baseline characteristics of these patients were similar to those in the overall Avelumab arm:
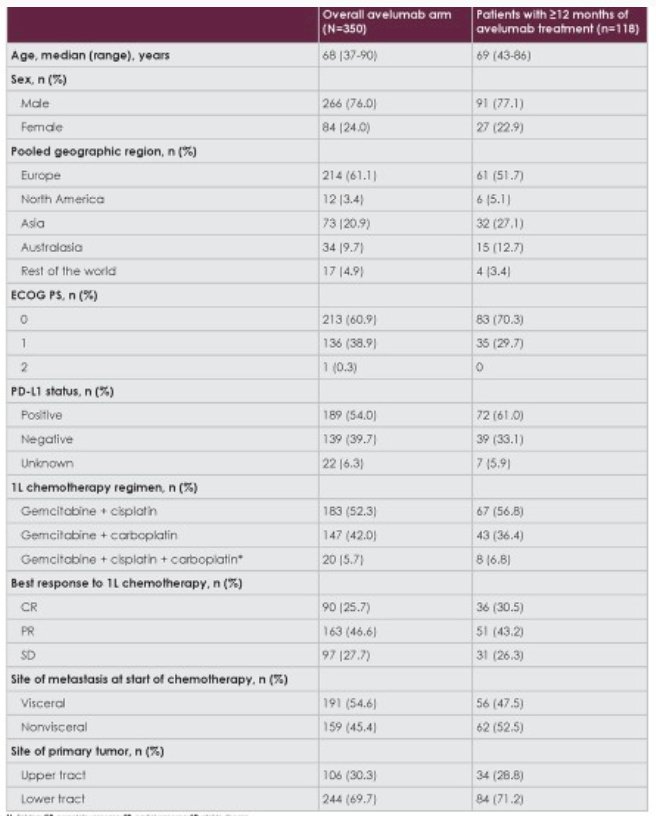
Long OS and investigator-assessed PFS were observed in patients who received >=12 months of Avelumab treatment. In patients who received >=12 months of Avelumab treatment, median OS was not reached (95% CI 50.9 months – not evaluable):

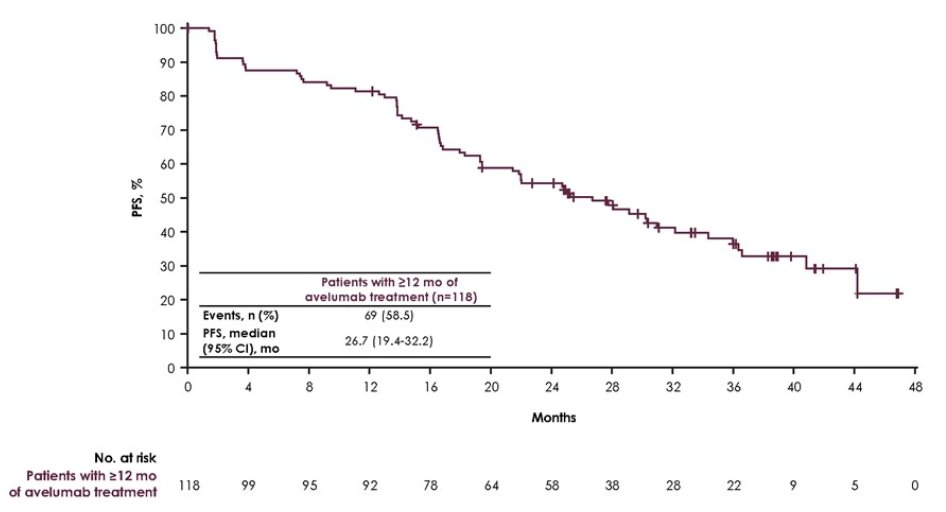
Median PFS was 26.7 months (95% CI 19.4-32.2):
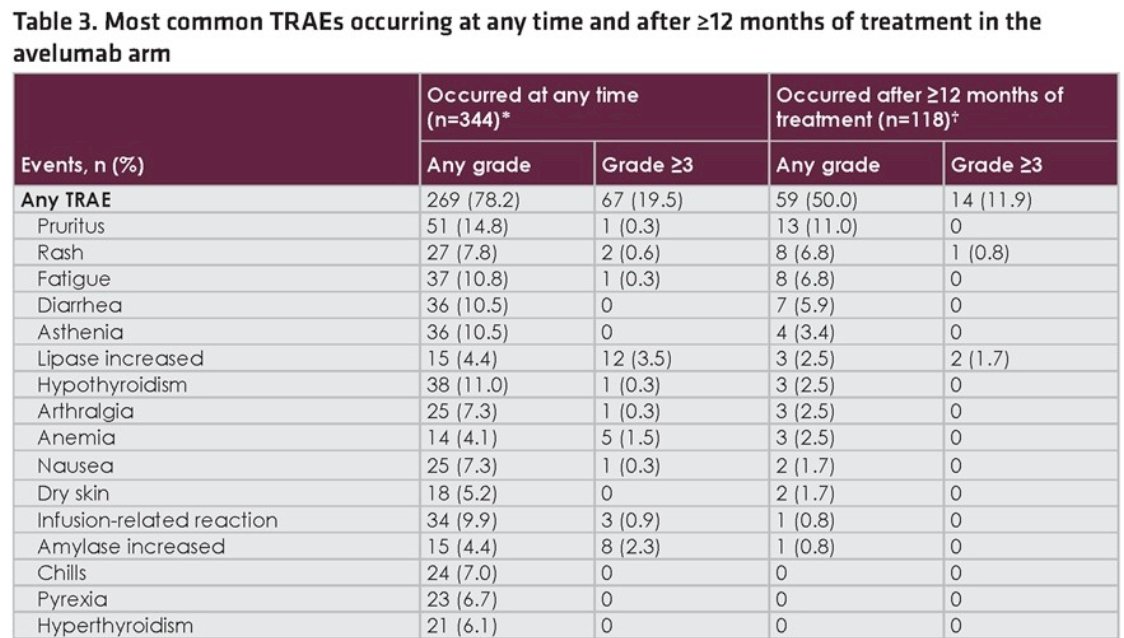
Among all treated patients in the overall Avelumab arm (n = 344), grade ≥3 treatment-related adverse events occurred in 67 (19.5%) and grade ≥3 immune-related adverse events occurred in 26 (7.6%). Among patients treated for ≥12 months (n = 118), grade ≥3 treatment-related adverse events occurred after ≥12 months in 14 (11.9%) and grade ≥3 immune-related adverse events occurred after ≥12 months in 5 (4.2%):
Dr. Aragon-Ching concluded her presentation discussing extended results of JAVELIN Bladder 100 for patients receiving ≥12 months of Avelumab first-line maintenance for advanced urothelial carcinoma with the following take-home messages:
- In the JAVELIN Bladder 100 trial, 33.7% of randomized patients in the Avelumab arm received >=12 months of treatment. In this subgroup, median OS was not reached and median PFS was 26.7 months
- Baseline characteristics of patients who received >=12 months of treatment were generally similar to those of patients in the overall Avelumab arm
- Prolonged Avelumab first-line maintenance treatment was associated with an acceptable safety profile that was consistent with prior Avelumab monotherapy studies, and no new safety signals were identified with longer treatment duration
- These results further support the use of Avelumab first-line maintenance until progression or unacceptable toxicity for all patients with advanced urothelial carcinoma that has not progressed with first-line platinum-based chemotherapy
Presented by: Jeanny B. Aragon-Ching, MD, GU Oncology, Inova Schar Cancer Institute, Fairfax, VA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:



