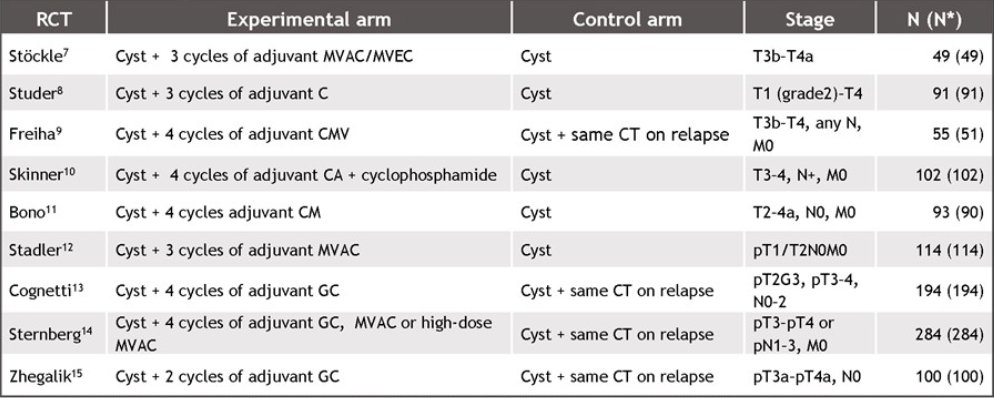
DFS and DMFS were assessed as potential surrogates for OS using individual patient data from 9 randomized controlled trials (median follow-up: 3.5-14.6 years) comparing cisplatin-based combination chemotherapy vs observation +/- deferred chemotherapy at relapse:
Strength of patient-level associations between DFS/DMFS and OS was measured by Spearman’s (ρ) rank correlation and derived from copula functions. Strength of trial-level associations between treatment effects on the surrogates and OS was measured by coefficient of determination (R2) and derived from regression, the robustness of which tested by cross-validation. The primary analyses used all patients (n = 1075) from all randomized controlled trials. Sensitivity analyses were performed with respect to trial follow-up and among lymph-node negative (N0) patients (n = 607).
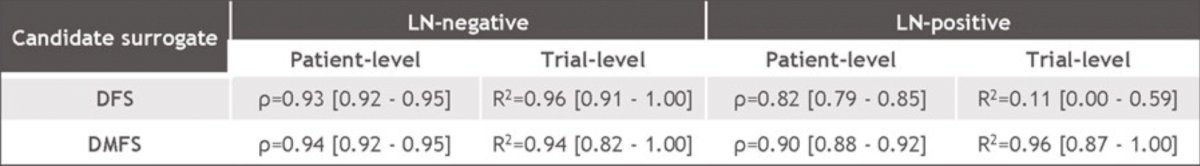
At the patient level, both DFS and DMFS were strongly correlated with OS, and at the trial level, correlation was moderate for DFS, but much stronger for DMFS. Trial-level associations between DFS and OS (A & B), and between DMFS and OS (C & D) for the entire study population (A & C), and lymph node-negative patients (B & D) are as follows:

For both surrogates, truncating events beyond 7 years had a modest impact on both correlations (<0.01 change in ρ, <0.05 change in R2, with narrower 95% CIs):

whereas limiting the focus to the N0 subgroup of patients strengthened both correlations:
The regression model’s 95% prediction intervals for OS hazard ratios contained the observed OS hazard ratios for all randomized controlled trials in cross-validation. Dr. Sternberg noted several limitations associated with this study including (i) being limited by the relatively low number of randomized trials each of which had relatively small sample sizes. The wide uncertainty in the estimates of HRs for DFS and OS due to sample size issues led to large confidence intervals around the estimated associations between treatment effects on DFS/DMFS and on OS; (ii) most randomized trials in the evidence base were conducted prior to the recent changes in the metastatic treatment landscape due to emergence of novel immunotherapy agents which could influence the surrogacy relationships; (iii) individual patient data could not be obtained for two published and one unpublished eligible trials that could expand the evidence base.
Dr. Sternberg concluded her presentation by discussing DFS and DMFS as surrogates for OS in adjuvant treatment of muscle-invasive bladder cancer with the following take-home messages:
- DFS had a moderated-to-strong association with OS in patients with muscle-invasive bladder cancer treated with adjuvant cisplatin-based chemotherapy. At the trial level, the measures of association were subject to more uncertainty due to wide confidence intervals and high variability of the estimates of hazard ratios of DFS and OS across the studies in the evidence base
- The trial-level association between DFS and OS was estimated to be stronger in lymph node-negative patients than in lymph node-positive patients
- DMFS had strong patient-level and trial-level associations with OS for the overall study population and for both subgroups defined by lymph node status
- The strengths of the patient-level and trial-level associations observed in this study were similar to those in recent studies in adjuvant treatment settings investigating the validity of intermediate endpoints as surrogates for OS
- The proposed surrogacy equations can assist predictions of OS benefit from the observed DFS and DMFS benefits in a prospective adjuvant muscle-invasive trial, however, the predictions from the DFS would be subject to higher uncertainty than those from DMFS and therefore should be approached with caution
Presented by: Cora Sternberg, MD, Englander Institute for Precision Medicine, Weill Cornell Medicine, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.