In the VESPER trial, 493 patients received dose-dense Methotrexate, Vinblastine, Doxorubicin and Cisplatin (dd-MVAC) or Gemcitabine and Cisplatin (GC) after randomization (NCT01812369). In this analysis, the authors limited the cohort to patients treated in neoadjuvant setting. They performed 3’ mRNA sequencing on TURB FFPE tissue, taking multiple samples when morphology and/or multiplexed GATA3 Cytokeratin 5/6 TUBB2a immunostaining highlighted distinct patterns. Consensus molecular subtype was then determined for each area from their transcriptomic profile.

The authors then assessed pathological response and 3-year (yr) progression-free survival (PFS) stratified according to the consensus molecular subtypes.
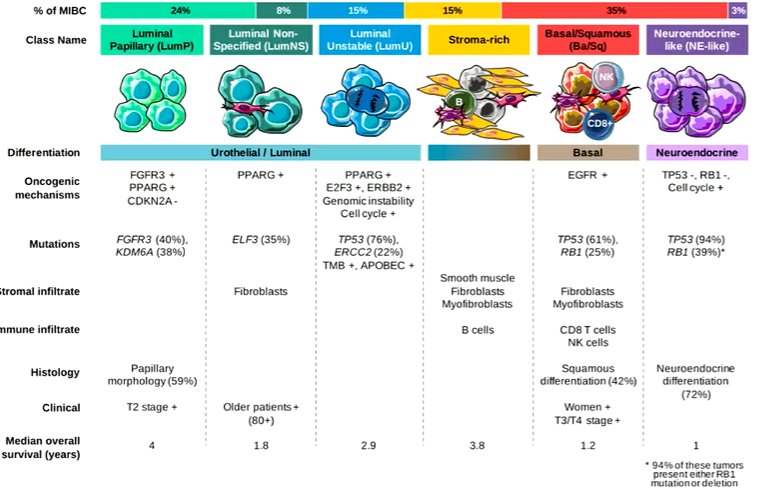
Among 296 cases, 97 presented intra-tumor immunostaining heterogeneity. For 251 cases, one single molecular subtype was detected within the tumor, including 37 luminal papillary, 60 luminal unstable, 17 luminal non-specified, 53 stroma-rich, 81 basal/squamous, and 3 neuroendocrine-like. 45 cases were mixed including 2 or more molecular subtypes (27 with basal/squamous component).
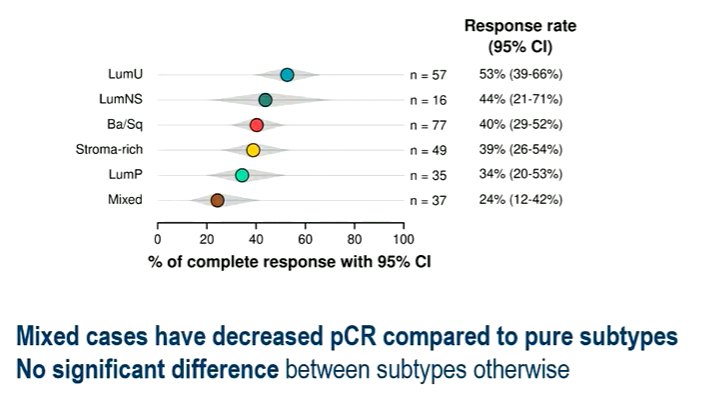
The authors did not identify a statistically significant difference in pathological response between pure molecular subtypes, though pathologic response was decreased for the mixed cases (OR=0.43, 95% CI 0.19-0.96, p=0.040).
Compared to luminal and stroma-rich subtypes, 3-year PFS was significantly decreased when basal/squamous subtype was detected, either pure or admixed with another molecular type (HR=2.16, 95% CI 1.46-3.20, p<1e-3).
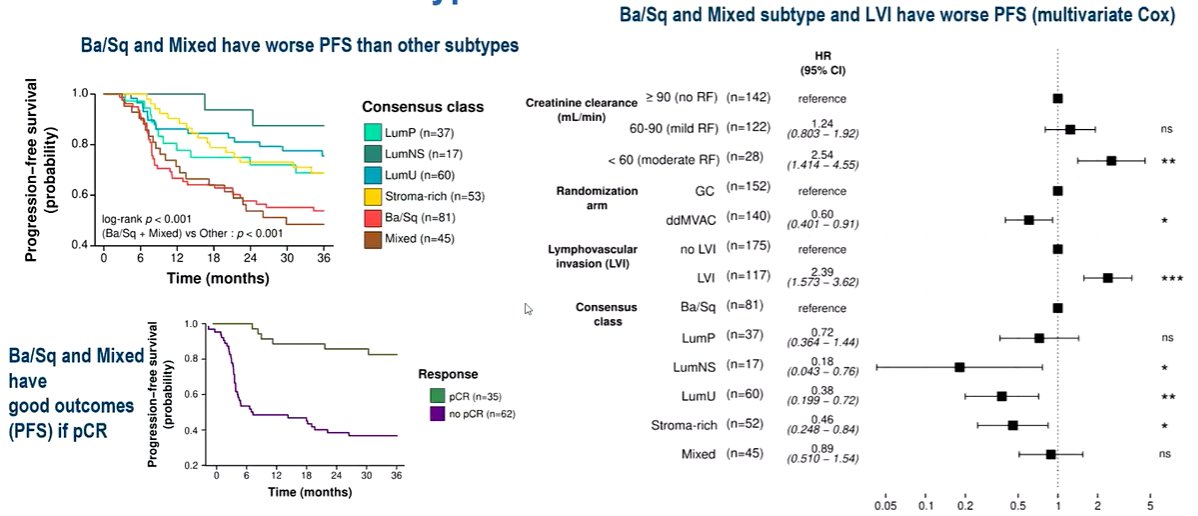
Lymphovascular invasion was also found to be a significant risk factor for 3yr PFS (HR=1.91, 95% CI 1.29-2.83, p=0.001), independently from molecular subtype.
Thus, the authors concluded that the basal/squamous molecular subtype (pure or mixed) is associated with a decreased 3-year PFS for patients treated with neoadjuvant chemotherapy in comparison with other subtypes. Additionally, they note that approximately 15% of patients have subtype spatial heterogeneity and this intra-tumor heterogeneity predicts a worse outcome after neoadjuvant chemotherapy.
Presented by: Clarice D. Groeneveld, Institut Curie and Centre de Recherche des Cordeliers, Paris, France
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.


