(UroToday.com) The 2022 ESMO annual meeting included a session focusing on multi-disciplinary updates in biomarkers in urinary cancers and a presentation by Dr. Elisabeth de Vries discussing the latest imaging advances in PET and beyond translated to bladder and kidney cancers. Dr. de Vries notes that molecular imaging takes advantage of tumor biology in vivo and numerous targets for ligands. With regards to PET imaging and translation to bladder and kidney cancers, the potential aim is to predict who will respond to what treatment and PET imaging. For bladder cancer, this includes HER2 (an antibody drug conjugate), immune checkpoint inhibitors, and immune response with CD8 imaging. For kidney cancer, this includes angiogenesis inhibitors, immune checkpoint inhibitors, and immune response with CD8 imaging.
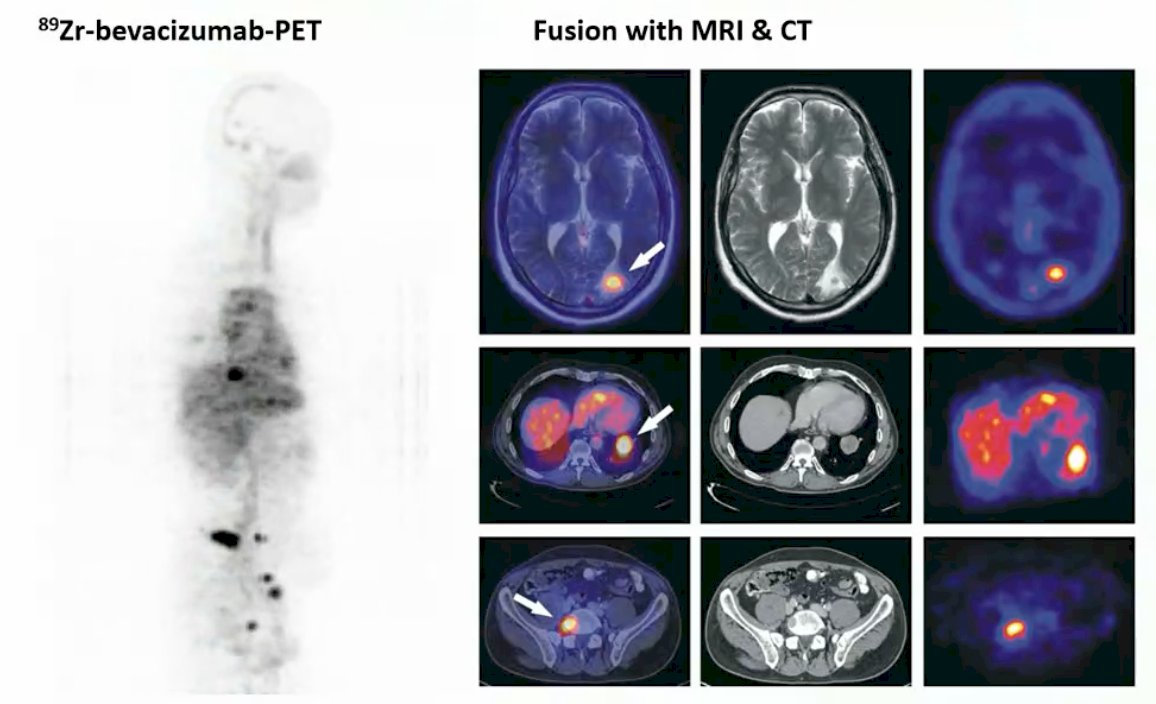
Dr. de Vries notes that ImmunoPET in oncology means imaging with antibodies. Antibodies are highly specific for target recognition and binding, offer essential treatment options in oncology, have a long elimination half-life, several days required for optimal target levels, and they are easy to label with radionuclides. Several examples for imaging include 89Zr-trastuzumab and 111In-trastuzumab-SPECT. Additionally, 89Zr-bevacizumab-PET in renal cancer has also been described:
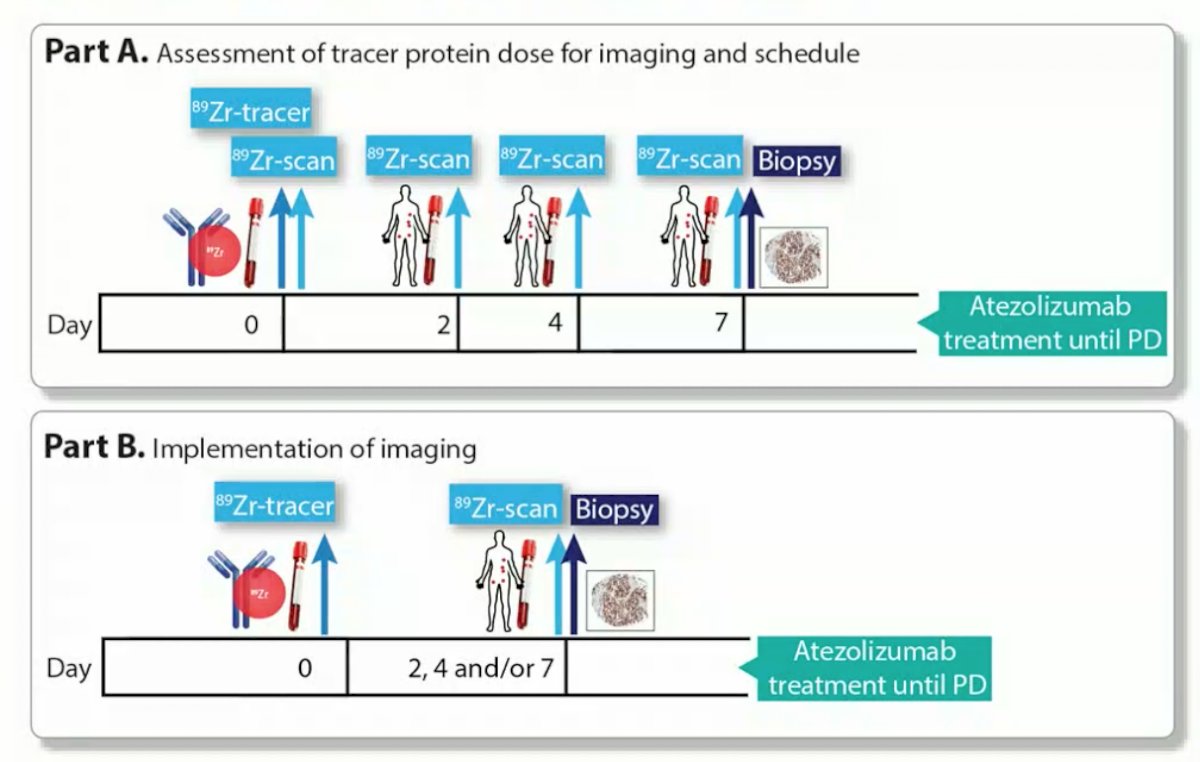
With regards to 89Zr-labeled PD-L1 antibodies in patients, they have the opportunity show responses in IHC negative tumors. Additionally, a biopsy provides only information about a small part of one tumor lesions where as 89Zr-labeled PD-L1 antibodies have the ability to disregard potential heterogeneity. Dr. de Vries noted the following trial design for an 89Zr-atezolizumab PET study:
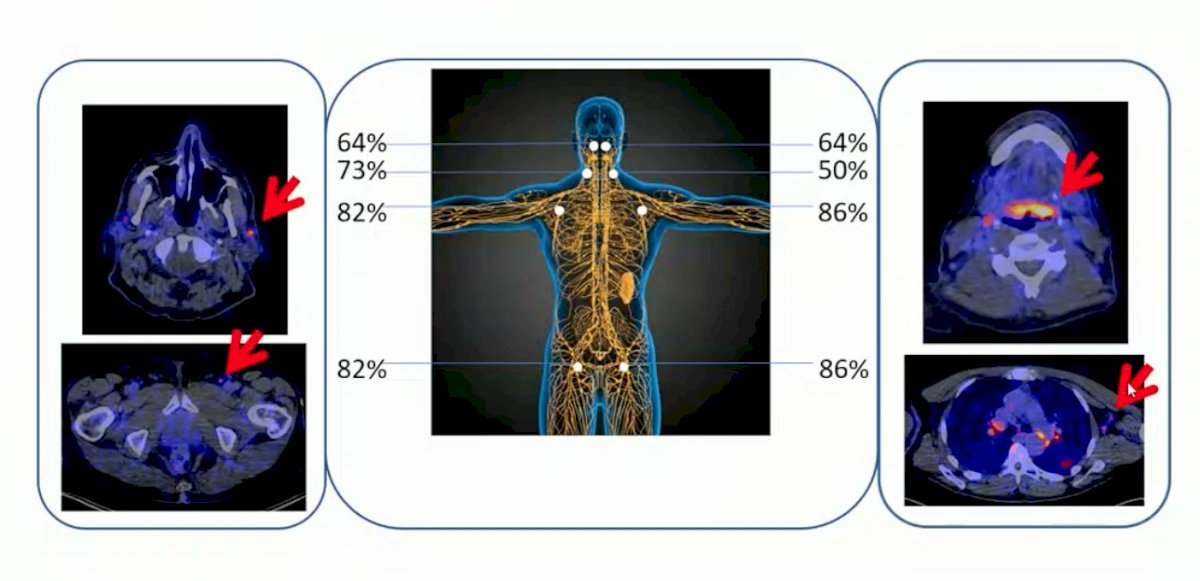
In this trial there were 22 patients across three tumor types (n = 9 bladder cancer) that were imaged before the start of atezolizumab therapy. The PET signal, a function of tracer exposure and target expression, was high in lymphoid tissues and at sites of inflammation:
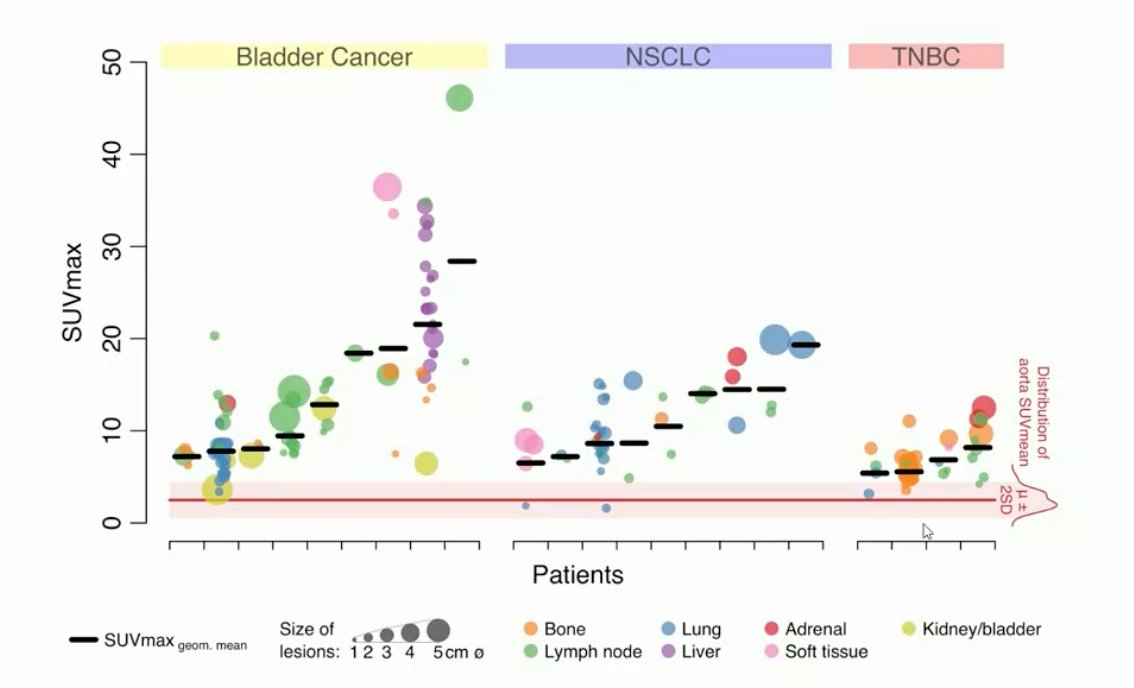
In tumors, uptake was generally high but heterogeneous, varying within and among lesions, patients, and tumor types:
Interestingly, clinical responses in these patients were better correlated with pretreatment PET signal than with immunohistochemistry- or RNA-sequencing-based predictive biomarkers, encouraging further development of molecular PET imaging for assessment of PD-L1 status and clinical response prediction.
Dr. de Vries then discussed an 89Zr-pembrolizumab study, which was published earlier in 20222:
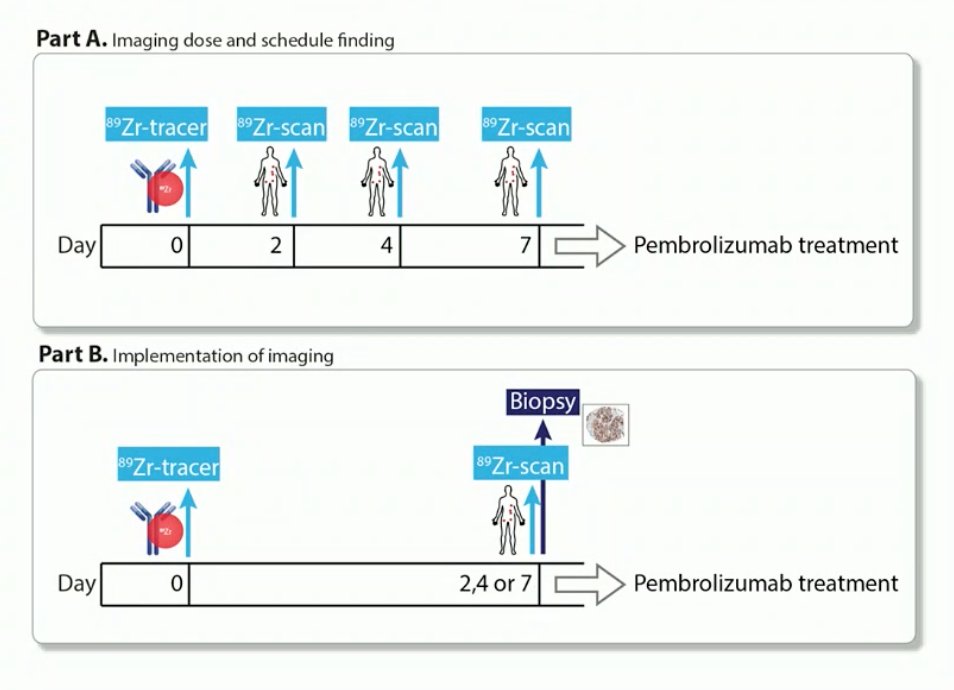
This study included day 7 uptake of 89Zr-pembrolizumab in NSCLC and melanoma:
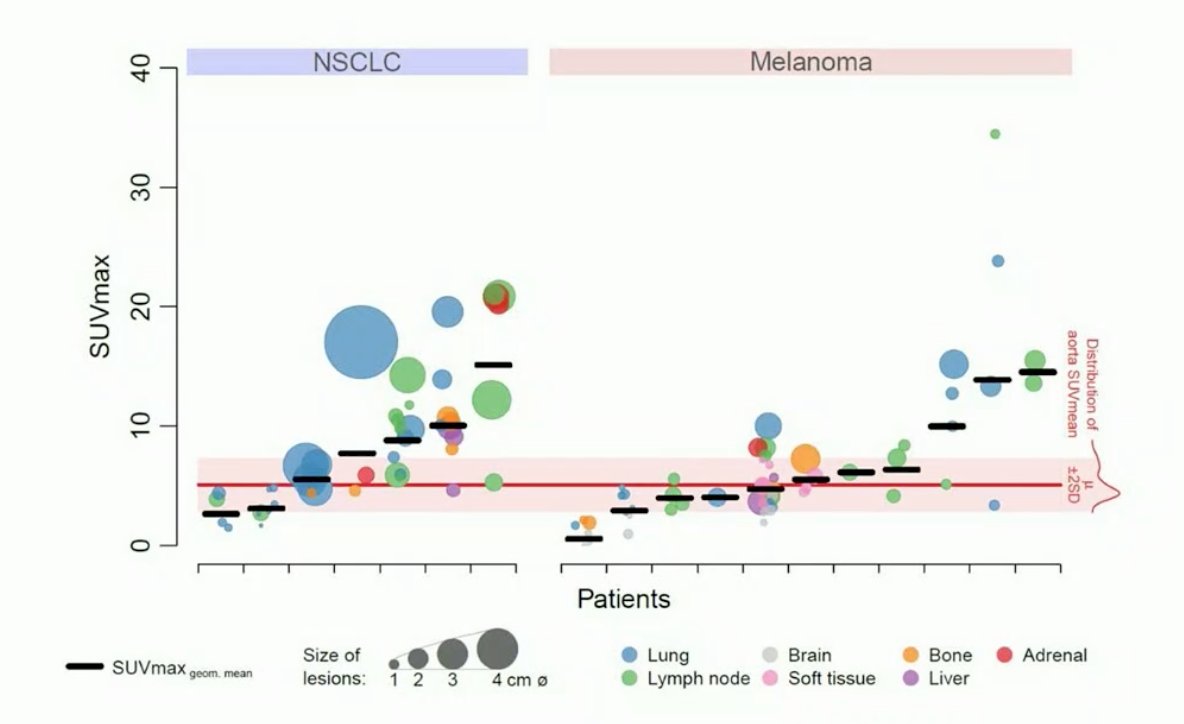
Dr. de Vries concluded her presentation by discussing the latest imaging advances in PET and beyond translated to bladder and kidney cancer with the following take home messages:
- This technology allows visualization of drug distribution and tumor characteristics
- May predict tumor response, PFS, and OS
- Provides insight into tumor heterogeneity and the immune system
- This technology is still at its infancy and requires validation, cooperation, and further determination to assess if it is meaningful
Presented by: E. G. Elisabeth de Vries, MD, PhD, University Medical Center Groningen, Groningen, The Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.
References:
- Bensch F, van der Veen EL, de Hooge MNL, et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat Med. 2018 Dec;24(12):1852-1858.
- Kok IC, Hooiveld JS, van de Donk PP, et al. 89Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann Oncol. 2022 Jan;33(1):80-88.


