HIF-2α is constitutively activated in RCC and is a transcription factor of multiple oncogenic pathways including TGF-α, GLUT1, cyclin D1, CXCR4, and VEGF. As has been recognized for decades now, VEGF, and its receptor, are key regulators of angiogenesis and are upregulated in RCC. Belzutifan, a first-in-class HIF-2α inhibitor, has shown antitumor activity and favorable safety in heavily pretreated advanced RCC and in patients with von Hippel-Lindau disease-associated tumors. Cabozantinib, a multikinase inhibitor that targets VEGF, is approved for advanced RCC.
In first line, mRCC, combination therapy has become standard of care. Thus, the authors designed this study to assess the use of belzutifan plus cabozantinib for treatment-naive patients with advanced RCC (NCT03634540).
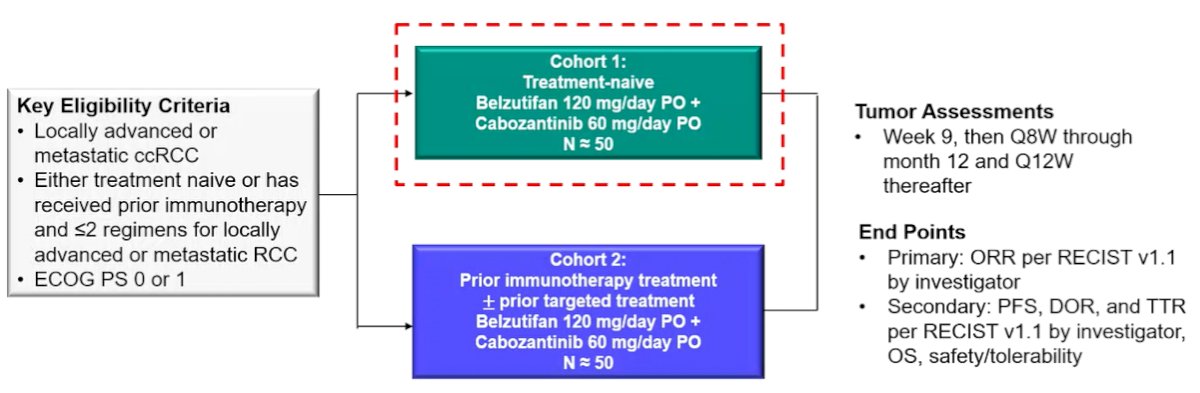
The authors enrolled treatment-naïve patients with advanced clear cell RCC and ECOG performance status of 0/1. In this phase 2 design, all patients received belzutifan 120 mg orally once daily plus cabozantinib 60 mg orally once daily. The primary endpoint was confirmed ORR (CR + PR) per RECIST v1.1 by investigator review with secondary endpoints of DOR, PFS, OS, and safety.
In cohort 1 of the trial, 35 patients were enrolled to date of a planned 50 patients. Of these, 10 (29%) discontinued treatment, primarily due to progressive disease (n=9). The median age of enrolled patients was 64 years (range, 33-89), and most had an ECOG PS of 0 (n=21; 60%). In terms of disease characteristics, most patients had IMDC favorable risk (n=21; 60%).
The median follow-up was 14.0 months (range, 0.2-33.0) and over that time a confirmed objective response was seen in 57% of patients with 2 CRs and 18 PRs. 13 patients (37%) had a best response of SD.

Among 35 evaluable patients, 33 (94%) had a reduction in target lesion volume. Among responders, the median duration of response was 28.6 months (range, 1.7+ to 28.6) and 13 patients had a response for 6 months or more. At the time of data cut-off, 25 patients had treatment ongoing. Stratified according to IMDC risk category, ORR was 62% among the 21 patients with favorable risk disease and 50% among the 14 patients with intermediate/poor risk.
The median progression-free survival was 30.3 months (95% CI, 9.4 to not reached) and the estimated 12-month PFS rate was 67%. To date, median OS was not reached and the estimated 12-month OS rate was 96%.

Among all treated patients, the most common any grade treatment-related AEs were anemia (n=25; 71%) and diarrhea (n=25; 71%). Grade 3 treatment-related AEs occurred in 13 (37%) and were most commonly due to hypertension (n=4; 11%) and fatigue (n=3; 9%). There were no grade 4 or 5 treatment-related AEs. One patient (3%) discontinued cabozantinib due to an AE (abdominal abscess) while no patients discontinued belzutifan due to an AE.

He specifically noted that anemia and hypoxia are distinct adverse events associated with belzutifan therapy. Treatment-related anemia may require either erythropoiesis-stimulating agents or transfusion. Similarly, treatment-related hypoxia may require supplemental oxygen or dose reduction.
Thus, Dr. Merchan concluded that these preliminary phase 2 data demonstrate that the combination of belzutifan plus cabozantinib has manageable safety with promising antitumor activity in treatment-naïve patients with advanced clear cell RCC.
Presented by: Jaime Merchan, MD, University of Miami Health System, Coral Gables, FLWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.


