(UroToday.com) On Monday, September 12, 2022, in Presidential Symposium III at the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Chouieri presented highly awaited results from the COSMIC-313 trial, examining the role of triplet therapy with cabozantinib, nivolumab, and ipilimumab as first-line treatment in patients with advanced renal cell carcinoma (aRCC).
There has been a dramatic evolution in the care of patients with aRCC in the past few years – combination therapy with an immune checkpoint inhibitor backbone has become standard of care. Among the available treatment options, cabozatinib inhibits tyrosine kinases including MET, VEGFR, and TAM kinases (TYRO3, AXL, MER) and promotes an immune-permissive environment, which may enhance response to checkpoint inhibitors. In the CheckMate-9ER trial, the combination of cabozanitinb and nivolumab was found to improve survival compared to sunitib and, as such, became standard of care. Nivolumab may also be combined with ipilimiumab in this setting based on data from CheckMate 214. In COSMIC-313 (NCT03937219), the authors evaluated the triplet combination of cabozantinib, nivolumab and ipilimumab, compared to cabozantinib, nivolumab and ipilimumab, as first-line treatment in patients with intermediate or poor risk advanced renal cell carcinoma (aRCC).
In this global, double-blind, randomized phase III study, the authors enrolled previously untreated patients with clear cell aRCC of IMDC intermediate or poor risk. Patients were randomized to receive cabozatinib 40 mg QD or matched placebo, stratified by region and IMDC risk. All enrolled patients, regardless of randomized group, received nivolumab (3 mg/kg IV Q3W) and ipilimumab (1 mg/kg IV Q3W) for 4 cycles followed by nivolumab (480 mg IV Q4W). Nivolumab was continued up to a maximum of 2 years.
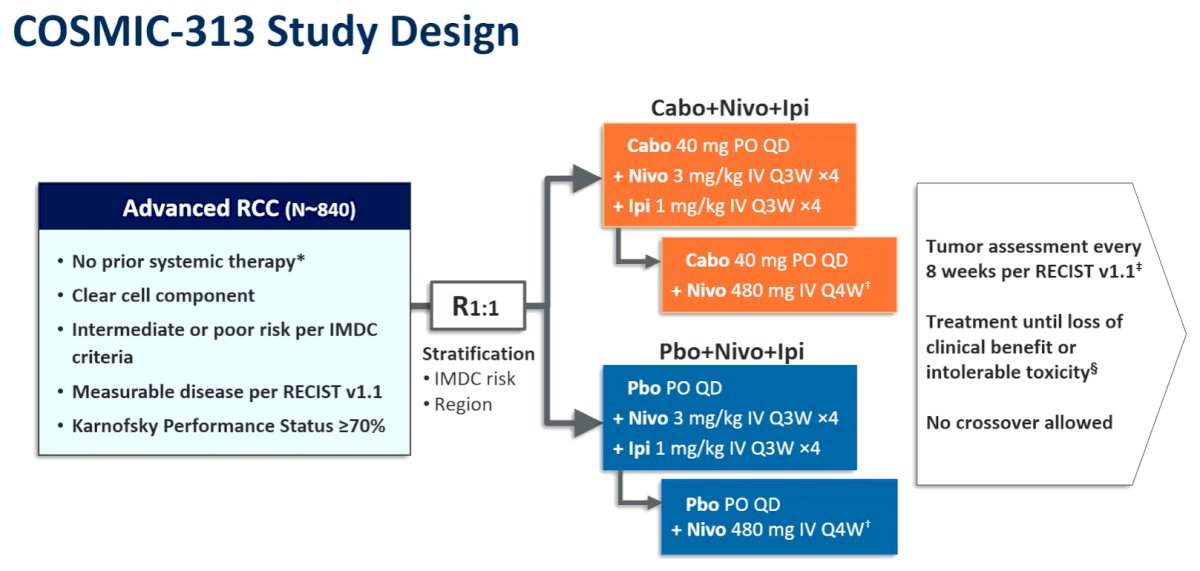
The primary endpoint was progression-free survival (PFS) by blinded independent radiology review per RECIST 1.1 in the first 550 randomized patients (PITT population). The secondary endpoint was overall survival (OS) in all randomized patients (ITT population); objective response rate (ORR) and safety were additional endpoints.
Between June 2019 and March 2021, 855 patients were randomized of whom 428 received cabozantinib, nivolumab and ipilimumab and 427 received placebo, nivolumab and ipilimumab. In the overall cohort, IMDC risk was intermediate for 75% and poor for 25%. In the PITT, population, the median follow-up is 20.2 months (16.1-31.3) and in the ITT population it is 17.7 months (10.2-31.3).
The study met the primary endpoint with a significantly improved PFS (HR 0.73, 95% CI, 0.57–0.94; p=0.013): median PFS was not reached (NR; 95% CI, 14.0–not estimable) among those receiving cabozantinib, nivolumab and ipilimumab as compared to 11.3 months (95% CI, 7.7–18.2) for placebo, nivolumab and ipilimumab.

In subgroup analyses, Dr. Choueiri highlighted that this effect was consistent across subgroup defined by age, sex, geographic region, performance status, prior nephrectomy, presence of sarcomatoid histology, and tumor characteristics. Notably, those with intermediate risk disease appeared to benefit more than those with poor risk disease per IMDC.

In the PITT population, the ORR was 43% (95% CI, 37.2–49.2) those receiving cabozantinib, nivolumab and ipilimumab as compared to 36% (95% CI, 30.1–41.8) for placebo, nivolumab and ipilimumab though the median duration of response was not reached in either group in either treatment group.

He then presented some data assessing the benefit of triplet intensification according to IMDC risk group. As highlighted in the figures below, there appears to be a greater benefit for the addition of cabozantinib in the intermediate risk group.

Grade 3/4 TRAEs somewhat more common in those receiving cabozatinib, nivolumab and ipilimumab (73%) than those receiving placebo, nivolumab and ipilimumab (41%). In each arm, three patients had grade 5 TRAEs. TRAEs leading to discontinuation of all treatment components occurred in 12% vs 5% of patients receiving cabozantinib, nivolumab and ipilimumab compared with placebo, nivolumab and ipilimumab, respectively.

Thus, Dr. Choueiri concluded that the triplet combination of cabozantinib, nivolumab and ipilimumab significantly improved progression-free survival compared to placebo, nivolumab and ipilimumab (a current standard of care) as first-line treatment of patients with IMDC intermediate or poor risk advanced RCC.
Presented by: Toni K. Choueiri, MD, Director, Lank Center for Genitourinary Oncology, Medical Director, International Strategic Initiatives, Jerome and Nancy Kohlberg Professor of Medicine at Harvard Medical School, Dana Farber Cancer Institute, Boston, MA
Written by: Christopher J.D. Wallis, University of Toronto, Twitter: @WallisCJD during 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


