(UroToday.com) The 2022 ESMO annual meeting included a session focusing on multi-disciplinary updates in biomarkers in urinary cancers and a presentation by Dr. Lisa Derosa discussing the importance of the microbiome. Dr. Derosa notes that we have identified a number of factors that impact tumor growth and response to cancer treatment, which now include polymorphic microbiomes. However, although the impact of the gut microbiome in genitourinary cancer immunotherapy was first studied, it was done serendipitously.
In a study led by Dr. DeRosa,1 they examined patients with advanced RCC and non-small-cell lung cancer treated with anti-PD-L1 mAb monotherapy or combination. Those receiving antibiotics within 30 days of beginning immune checkpoint inhibitors were compared with those who did not. Sixteen of 121 (13%) RCC patients received antibiotics, the most common were β-lactam or quinolones for pneumonia or urinary tract infections. In RCC patients, antibiotics compared with no antibiotics was associated with increased risk of primary progressive disease (75% versus 22%, P < 0.01), shorter progression free survival (median 1.9 versus 7.4 months, HR 3.1, 95% CI 1.4-6.9, p < 0.01), and shorter overall survival (median 17.3 versus 30.6 months, HR 3.5, 95% CI 1.1-10.8, p = 0.03):
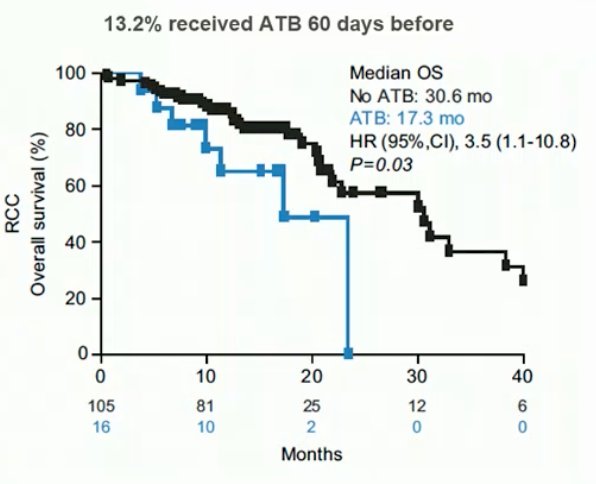
At ESMO 2021, Dr. Derosa presented the results of the phase 2 GETUG-AFU 26 NIVOREN trial and the impact of antibiotic therapy on outcomes. The GETUG-AFU 26 NIVOREN phase II trial (NCT 0301335) assessed the activity and safety of nivolumab in metastatic clear cell RCC patients who failed anti-angiogenic regimen. Patients who received antibiotics between 60 days before and 42 days after nivolumab initiation were compared with those who did not. Among 707 patients included in the study between February 2016 and June 2017, 104 (14.7%) received antibiotics. Median OS was 13.0 (95% CI 8.1-19.8; 67/104) months for antibiotic users versus 25.0 (95% CI 22.4-28.4; 284/603) months in non-users (HR 1.77, 95% CI 1.36-2.31):

Dr. Derosa notes that there are now 13 studies (encompassing 4,070 patients) confirming the negative impact of antibiotics in immunotherapy:

Dr. DeRosa also led a study published in European Urology [2], evaluating the predictive value of stool bacteria composition for immune checkpoint blockade efficacy among 69 advanced RCC patients. Recent antibiotic use (n = 11; 16%) reduced objective response rates from 28% to 9% (p < 0.03) and markedly affected the composition of the microbiota, facilitating the dominance of distinct species such as Clostridium hathewayi, which were also preferentially over-represented in stools from RCC patients compared with healthy volunteers. In a study of 338 patients with NSCLC treated with first- or second-line immune checkpoint inhibitors, Dr. Derosa’s group prospectively validate the predictive value of fecal Akkermansia muciniphila.3 They found that baseline stool Akkermansia muciniphila was associated with increased objective response rates and overall survival in multivariate analyses, independent of PD-L1 expression, antibiotics, and performance status. Intestinal Akkermansia muciniphila was accompanied by a richer commensalism, including Eubacterium hallii and Bifidobacterium adolescentis, and a more inflamed tumor microenvironment in a subset of patients. However, antibiotic use (20% of cases) coincided with a relative dominance of Akkermansia muciniphila above 4.8% accompanied by the genus Clostridium, both associated with resistance to immune checkpoint inhibitors.
Dr. Derosa notes that harmful bacteria contain members belonging to the Enterocloster genus, and Streptococcacae and Lactobacillaceae families already described in dismal prognosis immunoresistant patients:

Dr. Derosa then briefly discussed the tumor microbiota. Goubet et al.4 profiled tumor and blood samples, finding that follicular helper CD4+ T cells (TFH) are among the best therapeutic targets of pembrolizumab correlating with progression-free survival. TFH were associated with tumoral CD8 and PD-L1 expression at baseline, and the induction of tertiary lymphoid structures post-pembrolizumab. Blood central memory TFH accumulated in tumors where they produce CXCL13, a chemokine found in the plasma of responders only. Finally, TFH and IgG directed against urothelium invasive Escherichia coli dictated clinical responses to pembrolizumab in three independent cohorts.
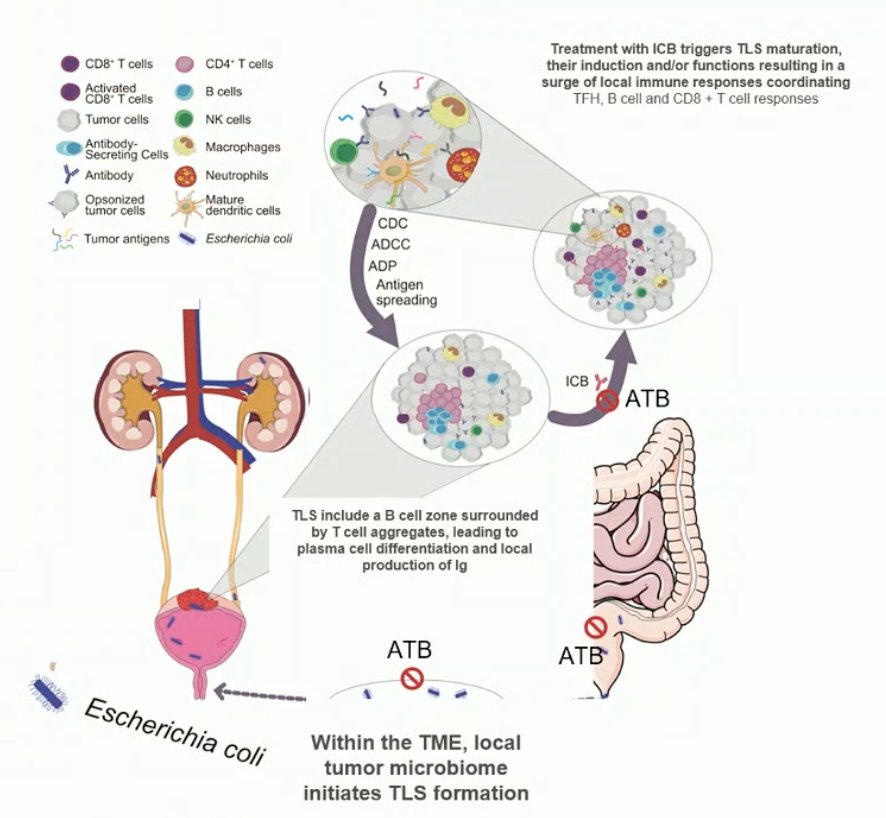
Dr. Derosa concluded her presentation discussing the microbiome with the following take-home messages:
- Antibiotic shift the gut microbiota composition
- Gut microbiota composition is associated with immunotherapy response/resistance
- The focus needs to be on community ecology and not on species
- Tumor microbiome represents a novel biomarker of immunotherapy response
- New strategies to guide oncological treatments, help stratifying patient populations amenable to immunotherapy, to ascribe their intestinal dysbiosis, and to better guide compensatory microbiota-centered interventions are needed
Presented by: Lisa Derosa, Institut Gustave Roussy, University of Paris Saclay, Villejuif, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.
References:
- DeRosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018 Jun 1;29(6):1437-1444.
- DeRosa L, Routy B, Fidelle M, et al. Gut Bacteria Composition Drives Primary Resistance to Cancer Immunotherapy in Renal Cell Carcinoma Patients. Eur Urol. 2020 Aug;78(2):195-206.
- Derosa L, Routy B, Thomas AM, et al. Intestinal akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat Med. 2022 Feb;28(2):315-324.
- Goubet AG, Lordello L, Costa Silva CA, et al. Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer. Cancer Discov. 2022 Aug 5;CD-22-0201.


