(UroToday.com) In the Prostate Cancer poster session of the European Society for Medical Oncology (ESMO) Annual Congress, Dr. Aggarwal provided preliminary results from a phase Ib study of a single dose of 177Lu-PSMA-617 followed by pembrolizumab in patients with metastatic castration-resistant prostate cancer (mCRPC). As monotherapy, Lu-PSMA-617 has demonstrated survival benefits in patients with mCRPC. However, immunotherapy (despite benefits in many other tumor types) has failed to demonstrate a benefit in this disease state. These authors sought to assess whether a single dose of 177Lu-PSMA-617 can induce an immunogenic priming effect to improve outcomes of men with mCRPC treated with pembrolizumab.
This phase 1b trial enrolled men with mCRPC who had progression on at least one prior androgen receptor (AR) signaling inhibitor (NCT03805594). Patients were required to have ≥ 3 PSMA-avid lesions on 68Ga-PSMA-11 PET. The authors did not perform genomic selection. Given the phase 1b nature of the study, the authors enrolled patients on one of 3 schedules: A) Single dose of Lu-PSMA-617 (7.4 GBq) followed by initiation of pembrolizumab (200 mg IV q 3 weeks); B) Lu-PSMA-617 x 1 dose given with first pembrolizumab administration; C) Lu-PSMA-617 x 1 dose given after initiation of pembrolizumab.
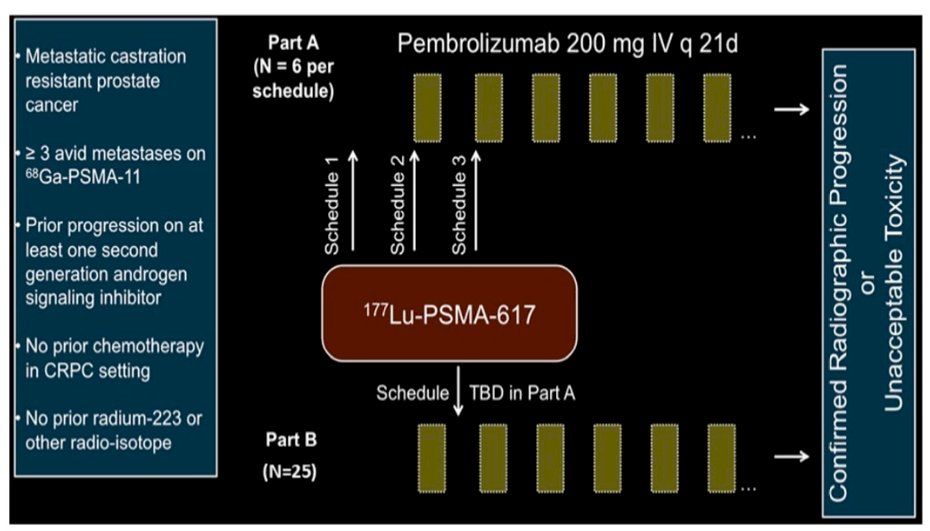
The primary endpoint was safety; secondary and correlative endpoints included objective response rate (ORR) by RECIST 1.1 criteria, PSA50 response rate, and whole blood immune profiling by CyTOF.
The authors enrolled 43 patients of whom 31 were treated on Schedule A, 6 on Schedule B, and 6 on Schedule C. The median age of enrolled patients was 71 (range 51 – 91). At baseline, 11 patients (26%) had visceral metastases and all were microsatellite stable with low TMB (< 10 mut/MB) on somatic genomic profiling.
In terms of prior therapy, 13 (33%), 8 (19%), and 22 (52%) patients had disease progression on abiraterone, AR antagonist, or both, respectively.
Following study treatment, there were no dose-limiting toxicities and one patient (2%) with a Grade ≥ 3 treatment-related adverse event (AE) (inflammatory arthritis, schedule B).
In terms of efficacy, the ORR was 48% (20/42 evaluable patients) and the median duration of response was 9.9 months (range: 2.5 to more than 18.3 months). Seven patients remain on study treatment with durations ranging from 3.5 to more than 20.5 months with durable responses.
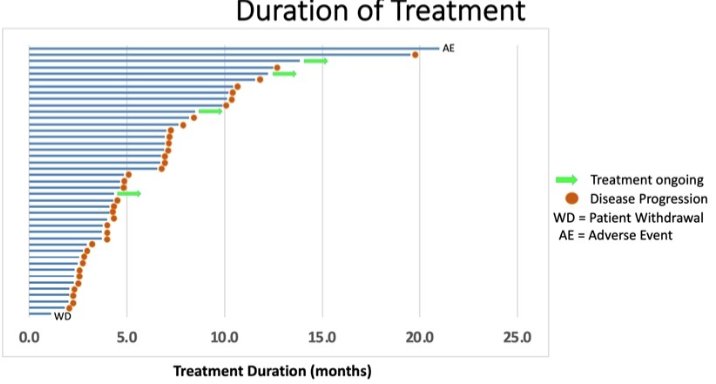
Median radiographic progression-free survival was 6.5 months (95% CI: 2.5 – 9.8). PSA50 and PSA90 response rates were 44% and 16%, respectively. Schedule A was the recommended phase 2 schedule based on aggregate safety and efficacy data.
In terms of secondary predictive analyses, serial profiling of immune cell subsets distinguished clinical responders from non-responders.
Thus, Dr. Aggarwal concluded that the approach of a single priming dose of 177Lu-PSMA-617 followed by pembrolizumab demonstrates a favorable safety profile and encouraging anti-tumor activity. Thus, this approach deserves further study.
Presented by: Rahul Aggarwal, MD, Genitourinary oncologist, UCSF Health, San Francisco, CAWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
Related Content:


