SPLASH is a multi-national, open-label study that commenced with a 27-patient lead-in prior to the randomized trial. In SPLASH, the authors enrolled patients with tumors exhibiting high-PSMA uptake on positron emission tomography-computed tomography (PSMA PET/CT) per blinded independent central review (BICR) who had not received chemotherapy for CRPC but who had progressed on an ARAT. Finally, patients had to have adequate bone marrow and end-organ reserve.
Patients received up to four cycles of 177Lu-PNT2002 at 6.8 GBq per cycle every 8 weeks and were followed for key endpoints of radiographic progression-free survival (rPFS) per BICR, overall survival, PSA response, dosimetry, and safety.
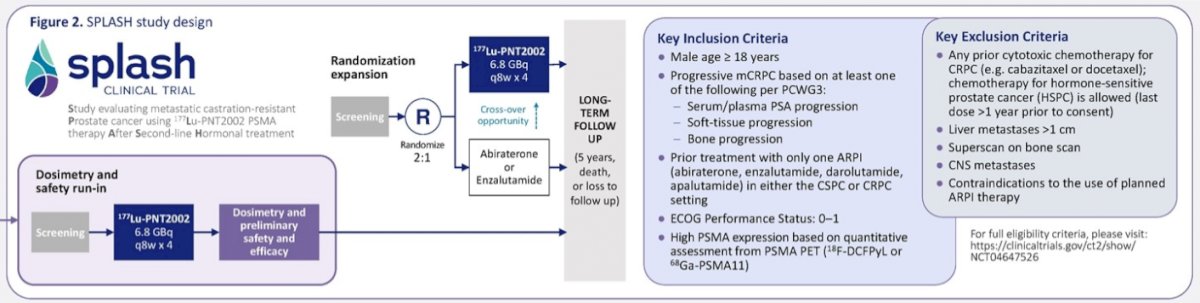
In this initial lead-in phase, 33 men underwent PSMA PET/CT to identify 27 (81.8%) eligible for treatment, of which 5 (15.2%) failed due to PSMA avidity criteria.
Among enrolled patient, included patients received a median 4 cycles of 177Lu-PNT2002, with a median dose of 6.9 (6.2-7.5) GBq/cycle. Six (22%) enrolled patients had previously received taxane chemotherapy for hormone-sensitive disease.
Based on a median rPFS follow-up of 9.2 months at data cut-off, median rPFS was 11.5 months with Kaplan Meier estimated 12-month rPFS of 44%. Notably, with a median follow-up of 11.7 months, median overall survival has not been reached.
One death was reported (non-treatment related) and 11 (42%) patients achieved a PSA50 response.

In terms of tolerability, grade ≥3 treatment emergent adverse events (TEAEs) occurred in 8 (29.6%) patients, of which anaemia (3, 11.1%) and haematuria (3,11.1%) occurred in >10% of patients. Treatment-related TEAEs in > 10% patients included dry mouth (7, 25.9%), nausea (5, 18.5%), fatigue (5, 18.5%), haematuria and anaemia (3, 11.1%).

Dr. Hansen thus concluded that, in mCRPC patients progressing following ARAT, 177Lu-PNT2002 was associated with a favorable rPFS and was well-tolerated. These data support the ongoing randomized portion of the SPLASH trial.
Presented by: Aaron R. Hansen, MD, Medical Oncologist, Princess Margaret Cancer Centre at UHN, Associate Professor of Medicine, University of Toronto, Toronto, Canada
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


