(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a presentation by Dr. Sarah Burdett discussing DADSPORT, a collaborative meta-analysis of aggregate data to assess duration of androgen suppression with post-operative radiotherapy. The DADSPORT meta-analysis collaboration conducted a systematic review and meta-analysis of results from NRG/RTOG 9601, GETUG-AFU 16, NRG/RTOG 0534, and the newly-available, RADICALS-HD trials to assess the effect of hormone therapy in men receiving radiotherapy following radical prostatectomy for localized prostate cancer.
Dr. Burdett and colleagues assessed the effects of adding hormone therapy to post-operative radiotherapy on overall survival (OS) and metastases-free survival (MFS), using published results and working with trialists to obtain additional data, including pre-publication results of RADICALS-HD. The primary analysis was a fixed-effect, inverse-variance meta-analysis, stratified by hormone duration (no hormone therapy, 6 months, and 24 months of hormone therapy) following radiotherapy. A pre-specified sensitivity analysis excluded men from NRG/RTOG 9601 who had PSA >1.5ng/ml at randomization. Important eligibility included trials that compared radiotherapy + hormone therapy versus radiotherapy following prostatectomy in men with intermediate/high risk localized disease, no evidence of regional or distant metastasis, and published or unpublished trials. As follows is a summary of the four trials included in the analysis:
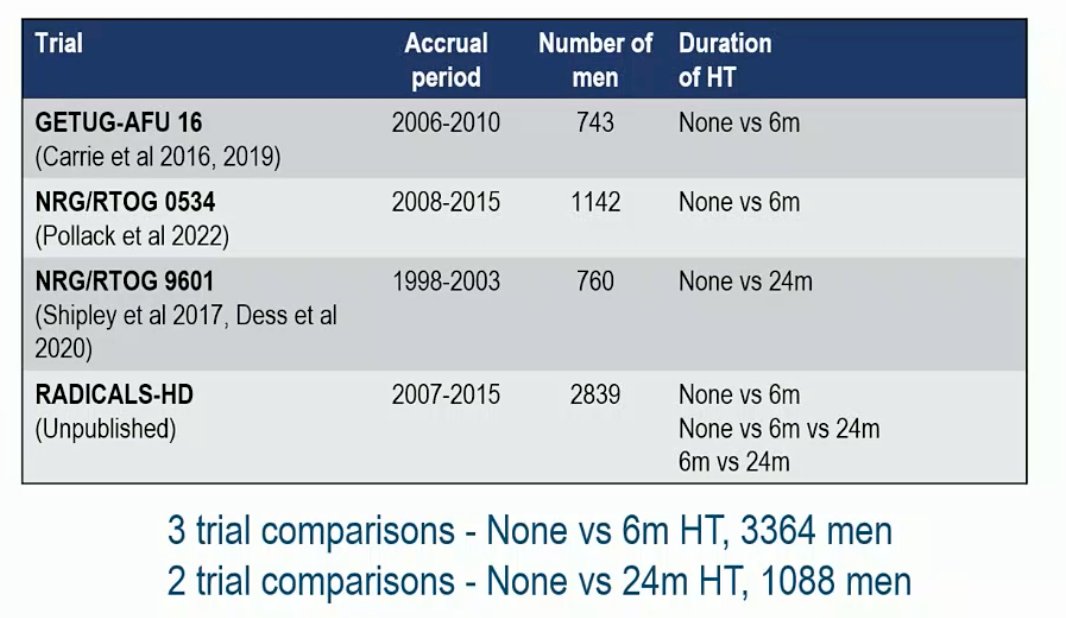
Among all 4 eligible trials (701 deaths, 4452 men; 100% of all randomized), the median age was 64 years in RTOG 0543, 65 years in RADICALS-HD & RTOG 9601, and 67 years in GETUG-16 (range 39-72). The majority of men were performance status 0/1, Gleason sum score < 8, no seminal vesicle involvement, with lower pT stage and fewer positive margins in GETUG-16 & RTOG 0543. There were 15% of men in RTOG 9601 that had pre-entry PSA of >1.5 to 4 ng/mL with a pre-planned sensitivity analysis to exclude these patients. The median follow-up was ≥8 years and there was no clear improvement in OS with hormone therapy compared to no hormone therapy (HR 0.87, 95% CI 0.75-1.01), irrespective of whether hormone therapy was 6 months or 24 months:
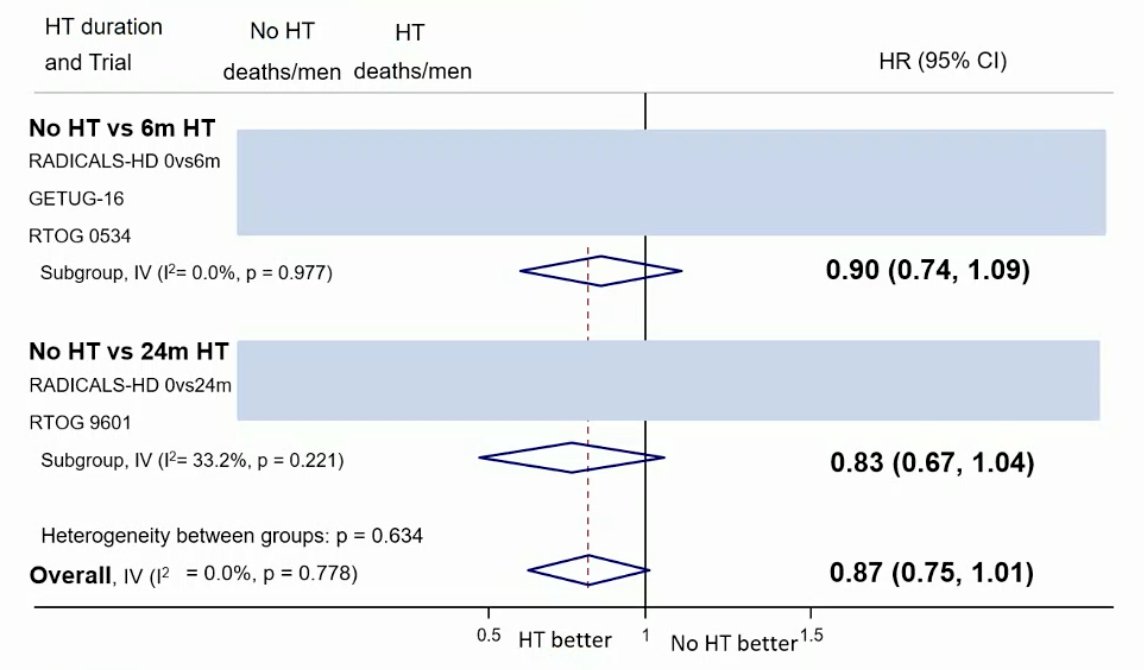
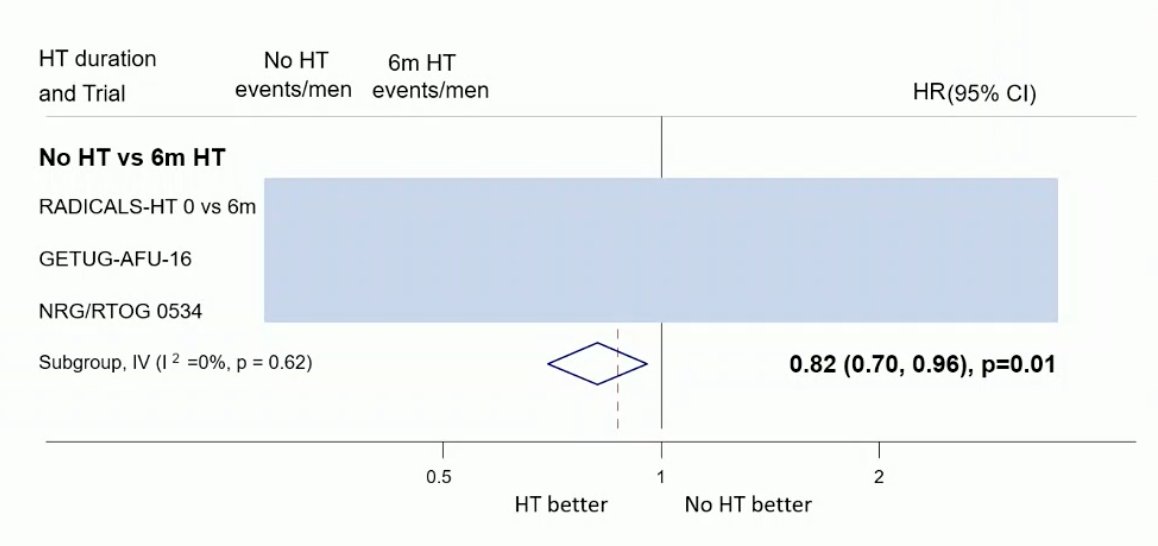
The results of a sensitivity analysis to exclude men with PSA >1.5ng/mL were also comparable to that of the primary analysis with no clear improvement in OS with hormone therapy compared to no hormone therapy (HR 0.91, 95% CI 0.78-1.06). Data on MFS from two trials of 24 months of hormone therapy vs no hormone therapy are not yet available. Based on data from three trials (653 events, 3364 men; 100%), there was evidence that 6 months of hormone therapy improved MFS compared to no hormone therapy (HR 0.82, 95% CI 0.70-0.96, p = 0.01), with a 5-year absolute improvement of 2% (CI 0% - 3%):
Based on a preliminary network meta-analysis in keeping with pairwise analysis, there was no clear evidence of difference in survival with either 6 or 24 months of hormone therapy versus none, and there was no clear evidence of a difference between 6 versus 24 months.
Dr. Burdett concluded her presentation discussing DADSPORT, a collaborative meta-analysis of aggregate data to assess duration of androgen suppression with post-operative radiotherapy by suggesting the following next steps to better define:
- The effect of hormone therapy on MFS and PCSS
- Any difference in the effect of hormone therapy due to radiotherapy delivery (pre-planned sensitivity analyses), salvage radiotherapy only (excluding adjuvant radiotherapy), and men who received radiotherapy to the prostate bed and lymph nodes (3rd arm, RTOG 0543)
- Whether any effect is consistent (or not) across pre-defined participant subgroups, including pre-surgical PSA, Gleason sum score, seminal vesicle involvement, surgical margins, CAPRA-S risk group, PSA level pre-RT, and cardiac comorbidity
- The relative effects of all durations of hormone therapy in network meta-analyses (all outcomes, all trial comparisons)
Presented by: Sarah Burdett, MRC Clinical Trials Unit at UCL, Institute of Clinical Trials and Methodology, London, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


