(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a discussant presentation by Dr. Nicolas Mottet discussing two key abstracts including “Duration of androgen suppression with post-operative radiotherapy (DADSPORT): A collaborative meta-analysis of aggregate data” presented by Dr. Sarah Burdett and “Quality of life and patient-relevant endpoints with darolutamide in the phase 3 ARASENS study” presented by Dr. Karim Fizazi.
Dr. Mottet notes that based on extended following up the RTOG 9601,1 GETUG-AFU 16,2 and NRG/RTOG 05343 trials, hormonal therapy during salvage radiotherapy does improve metastasis free survival:
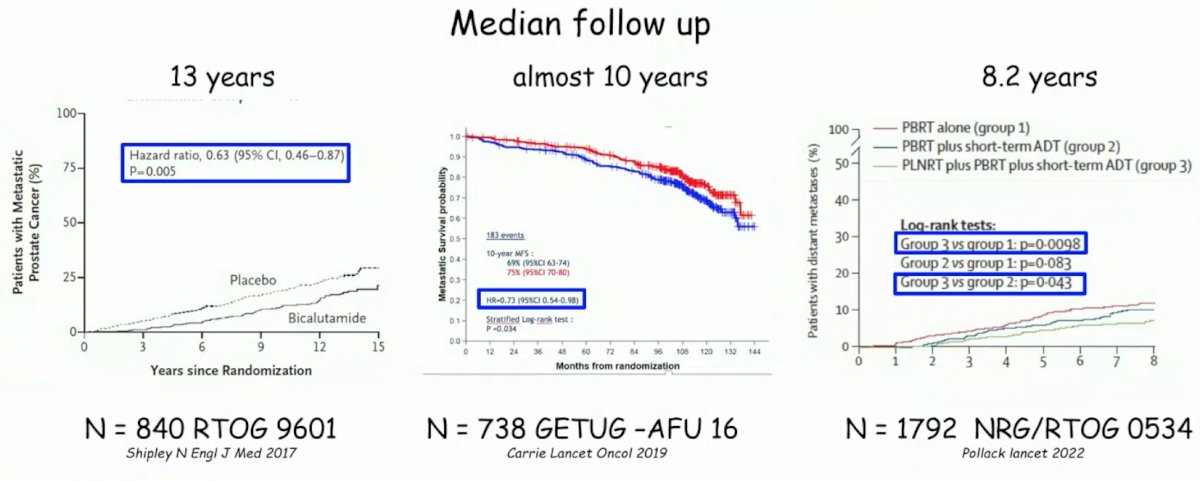
By the same token, among these three trials, hormone therapy has generally had little effect on overall survival:
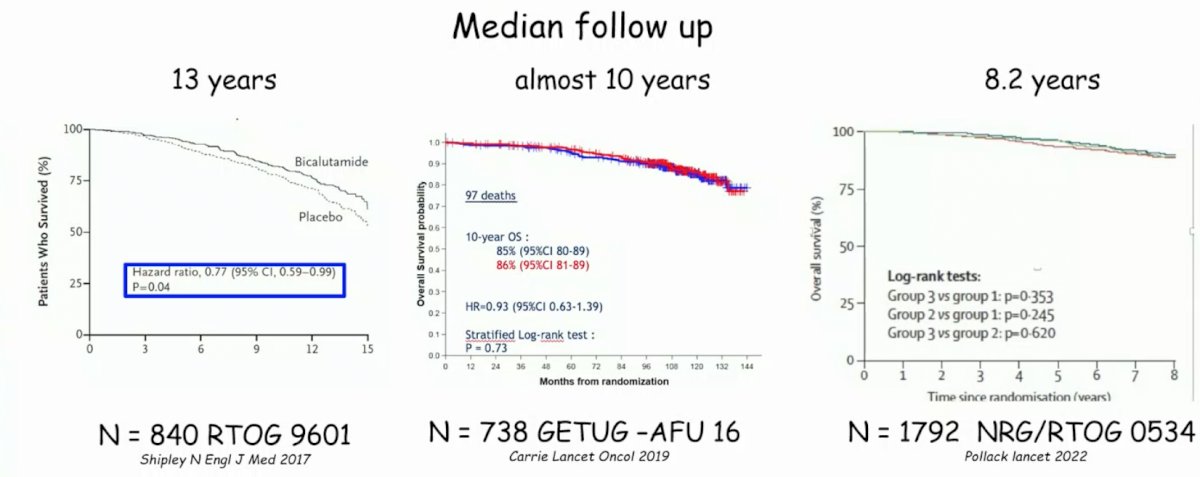
Generally, there is some benefit to adding hormone therapy to salvage radiotherapy, but with some added side effects, which are generally mostly acute and low-grade events. However, there are still some questions and limitations as highlighted by Dr. Mottet:
- For RTOG 9601: Radiotherapy (64.8 Gy) +/- bicalutamide (150 mg) for 24 months
- 12% of patients had a post-op PSA > 0.5 ng/mL
- Generally the median PSA at inclusion was high (0.6 ng/mL) with 53% of patients having a PSA < 0.7 ng/mL, 31% with a PSA 0.7-1.5 ng/mL, and 15% with a PSA of 1.5 – 4.0 ng/mL
- GETUG AFU-16: External-beam radiotherapy +/- 6 months of LHRH
- Undetectable post-op PSA
- Median PSA at inclusion was 0.3 ng/mL
- NRG/RTOG 0534: pelvic beam radiotherapy vs pelvic beam radiotherapy + 4-6 months ADT vs pelvic beam radiotherapy + whole pelvic radiotherapy + 4-6 months ADT
- Median PSA at inclusion was 0.35 ng/mL
From the work presented by Burdett et al. at ESMO 2022, based on data from three trials (653 events, 3364 men; 100%), there was evidence that 6 months of hormone therapy improved MFS compared to no hormone therapy (HR 0.82, 95% CI 0.70-0.96, p = 0.01), with a 5-year absolute improvement of 2% (CI 0% - 3%):

The median follow-up was ≥8 years and there was no clear improvement in OS with hormone therapy compared to no hormone therapy (HR 0.87, 95% CI 0.75-1.01), irrespective of whether hormone therapy was 6 months or 24 months:
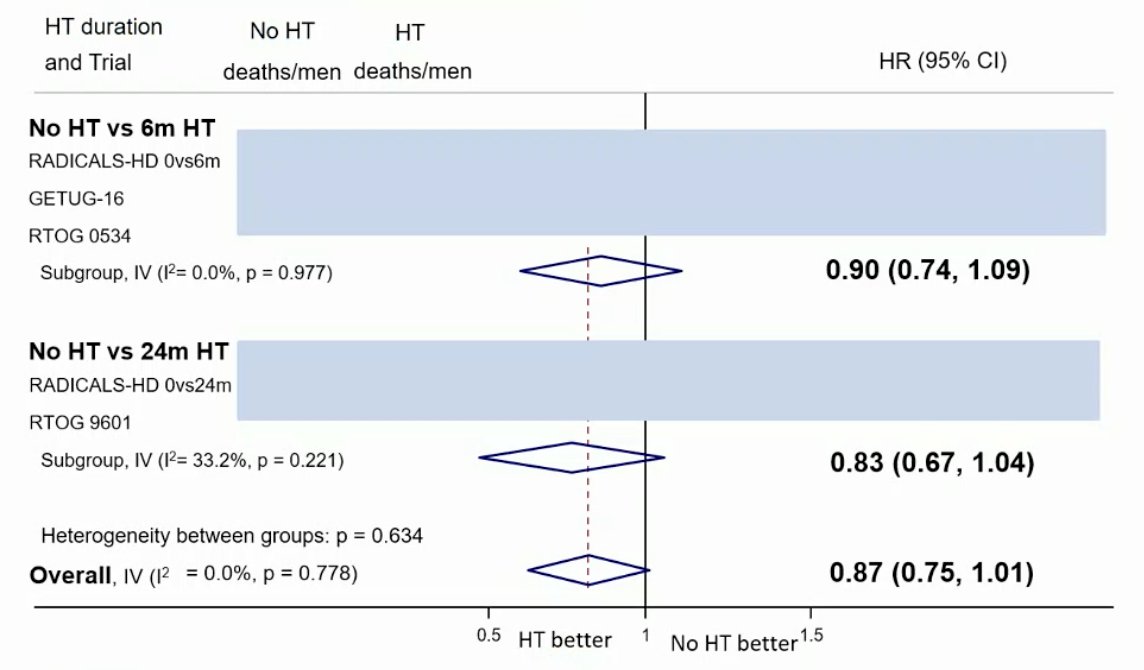
Dr. Mottet notes that there are several issues:
- Up front staging – no patients had the most sensitive modality, namely PSMA PET/CT (either 68Ga or 18F). Previous studies suggest that among patients in the biochemical recurrent setting that even at a PSA of 0.2-0.5 ng/mL, 37.9% of patients have a positive PSMA PET/CT, 16.9% had pelvic disease, and 21.0% had distant relapse
- Do all relapses need treatment? - the impact of relapse may be highly variable given that across studies hazard ratios range from 1.03 to 2.32 with regards to survival outcomes. In a systematic review assessing the prognostic value of biochemical recurrence following treatment with curative intent, Van den Broeck et al.4 define the EAU low-risk biochemical recurrence as a PSA-doubling time >1 year and pathologic ISUP grade <4 (whereby salvage treatment should be discussed but may not be needed), and EAU high-risk biochemical recurrence as a PSA-doubling time <= 1 year or pathological ISUP grade 4-5 (whereby salvage treatment is needed). Thus, is salvage after radiotherapy needed? Probably but not always. If yes when? As early as possible (therefore PSMA PET/CT is questionable). If ADT is added, the benefit is in improved metastases free survival (6 months) with no OS benefit after >8 years of follow-up.
Dr. Mottet then discussed the quality of life data for ARASENS, noting that standard of care for mHSPC is ADT + something (supported by all of the guidelines). For overall survival, the following options are available in the mHSPC setting:

Now, we also have the triplet therapies, specifically data from PEACE-15 and ARASENS6:

Dr. Mottet notes that the added side effects of triple therapy are minimal, with some increased risk of hypertension in PEACE-1 for patients receiving abiraterone compared to the control group. Based on data presented by Dr. Fizazi at ESMO 2022, there is no signal of major added side effects:
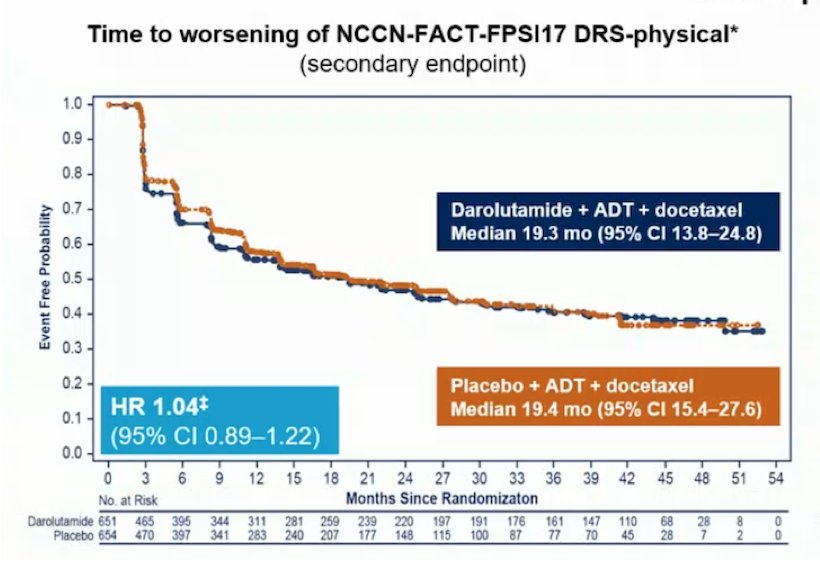

However, Dr. Mottet notes that based on data from Rush et al.7 and the STAMPEDE platform, quality of life (QLQ-C30), physical functioning, and social functioning are worse for docetaxel compared to abiraterone (particularly in the first 36 weeks of treatment). As such, this may be a limitation for both PEACE-1 and ARASENS.
Dr. Mottet concluded his discussant presentation by emphasizing that in 2022 we have:
- Multiple standard of care options for mHSPC, which is in all of the available guidelines and all based on ADT + something
- Triplet systemic therapy, which improves survival (darolutamide or abiraterone) compared to ADT + docetaxel
- Acceptable tolerance with no major extra toxicity outside of neutropenia, with quality of life maintained compared to ADT + docetaxel
- However, the real remaining question is: is triplet therapy useful for all or some M1? It is unlikely that we will ever have a randomized trial to answer this question, but perhaps with pooling of high level data we will be able to answer this question
Presented by: Nicolas Mottet, MD, PhD, Centre Hospitalo-Universitaire de Saint Etienne, Saint-Etienne, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med 2017;376(5):417-428.
- Carrie C, Hasbini A, de Laroche G, et al. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomized, multicentre, open-label phase 3 trial. Lancet Oncol 2016;17(6):747-756.
- Pollack A, Karrison TG, Balogh AG, et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): An international, multicentre, randomized phase 3 trial. Lancet. 2022 May 14;399(10338):1886-1901.
- Van Den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic Value of Biochemical Recurrence Following Treatment with Curative Intent for Prostate Cancer: A Systematic Review. Eur Urol 2019 Jun;75(6):967-987.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Rush HL, Murphy L, Morgans AK, et al. Quality of life in men with prostate cancer randomly allocated to received docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2022 Mar 10;40(8):825-836.


