This was a preplanned analysis of data from 1,172 men receiving ADT+/- docetaxel +/- abiraterone acetate + prednisone. The analysis required a PSA at 8 months from ADT initiation, with a data cut off of June 1, 2021. rPFS and OS were assessed overall, and then by randomization groups. The analysis was performed for the following 8-month PSA cut-offs: 0.2 and 4 ng/ml. Additionally, median values of rPFS and OS were calculated from 8 months-PSA using the Kaplan Meier method.
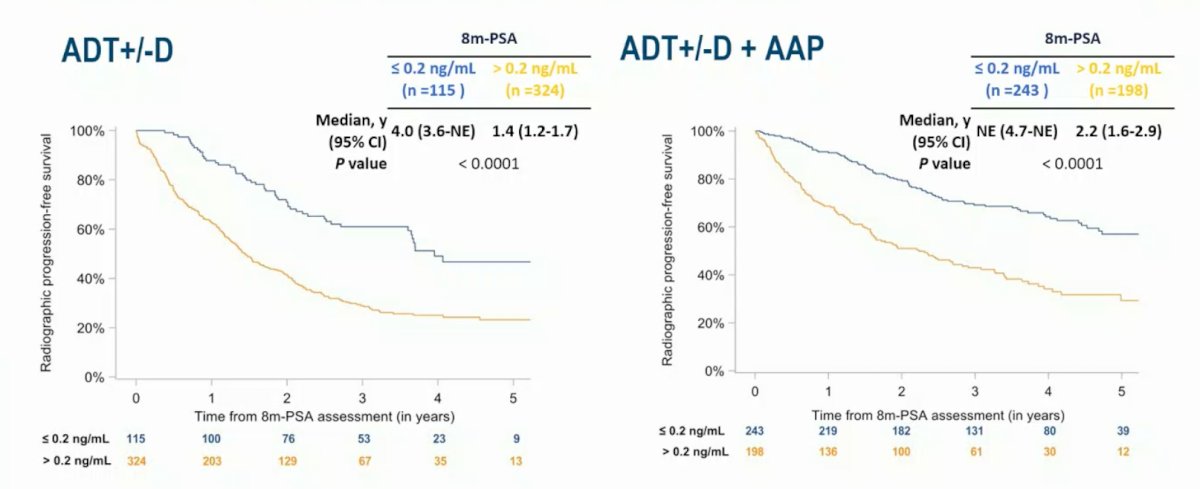
PSA values at 8 months after ADT initiation were available in 931 men (79%). Standard of care was ADT + docetaxel in 62%, and 56% had “high volume” disease. The median follow-up was 4.4 years (95% CI 4.3-4.5). In the overall population, 8 month PSA > 0.2 vs <= 0.2 ng/mL was associated with worse rPFS in the ADT +/- docetaxel (median 1.4 years, 95% CI 1.2-1.7 vs 4.0 years, 95% CI 3.6 to not evaluable, p < 0.0001) and the ADT +/- docetaxel + abiraterone acetate + prednisone arms (2.2 years, 95% CI 1.6 to 2.9 vs median not evaluable, 95% CI 4.7 to not evaluable, p<0.0001):
In the overall population, 8 month PSA > 0.2 vs <= 0.2 ng/mL was associated with worse OS in the ADT +/- docetaxel (median 3.5 years, 95% CI 3.1-4.1 vs not evaluable, 95% CI 4.8 to not evaluable, p<0.0001) and the ADT +/- docetaxel + abiraterone acetate + prednisone arms (3.4 years, 95% CI 2.8 to 3.9 vs not evaluable, 95% CI 5.7 to not evaluable, p<0.0001):

In the ADT + docetaxel population, 8 month PSA > 0.2 vs <= 0.2 ng/mL was associated with worse OS in the ADT + docetaxel (median 3.5 years, 95% CI 2.8-4.0 vs not evaluable, 95% CI 4.0 to not evaluable, p = 0.0007) and the ADT + docetaxel + abiraterone acetate + prednisone arms (3.6 years, 95% CI 2.9 to not evaluable vs not evaluable, 95% CI 4.7 to not evaluable, p<0.0001):

In the ADT + docetaxel population, 8 month PSA > 4 vs <= 4 ng/mL was associated with worse OS in the ADT + docetaxel (median 2.1 years, 95% CI 1.5 to 2.5 vs 4.5 years, 95% CI 4.0 to not evaluable, p < 0.0001) and the ADT + docetaxel + abiraterone acetate + prednisone arms (1.6 years, 95% CI 0.9 to 2.4 vs not evaluable, 95% CI 4.7 to not evaluable, p < 0.0001):

Dr. Mescam concluded her presentation discussing 8-month PSA outcomes of men with mCSPC in the PEACE-1 trial with the following take-home messages:
- 8-month PSA value strongly predicts both rPFS and OS in men with mCSPC in PEACE-1
- There was a significantly higher number of patients that had a PSA <= 0.20 ng/mL with the combination of ADT + docetaxel + abiraterone acetate + prednisone arms versus ADT + docetaxel
- Early therapeutic intervention in men with unfavorable 8-month PSA values needs to be investigated
- The clinical and molecular characteristics of the population with elevated 8-month PSA values deserve better understanding
Presented by: Gwenaelle Gravis Mescam, Department of Medical Oncology, Institut Paoli-Calmettes, Aix-Marseille Université, Marseille, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:


