In this analysis, overall survival (OS) was compared in subgroups of mHSPC patients (node +/- bone and bone-only), randomized 2:1 ADT +/- docetaxel or 1:1 ADT +/- abiraterone acetate and prednisolone. Nodal burden was dichotomized into low (<5 nodes) and high (≥5 nodes) burden. Cox models were stratified by time period and adjusted for N stage, age (<70, ≥70), WHO performance status, NSAID/aspirin use, radiotherapy, bone metastases, and CHAARTED low or high volume.
1,086 patients from ADT +/- docetaxel and 990 patients from ADT +/- abiraterone acetate and prednisolone were studied. CT/MRI scans for 373 ADT +/- docetaxel and 602 ADT +/- abiraterone acetate and prednisolone patients were also centralized and reviewed for nodal metastases:
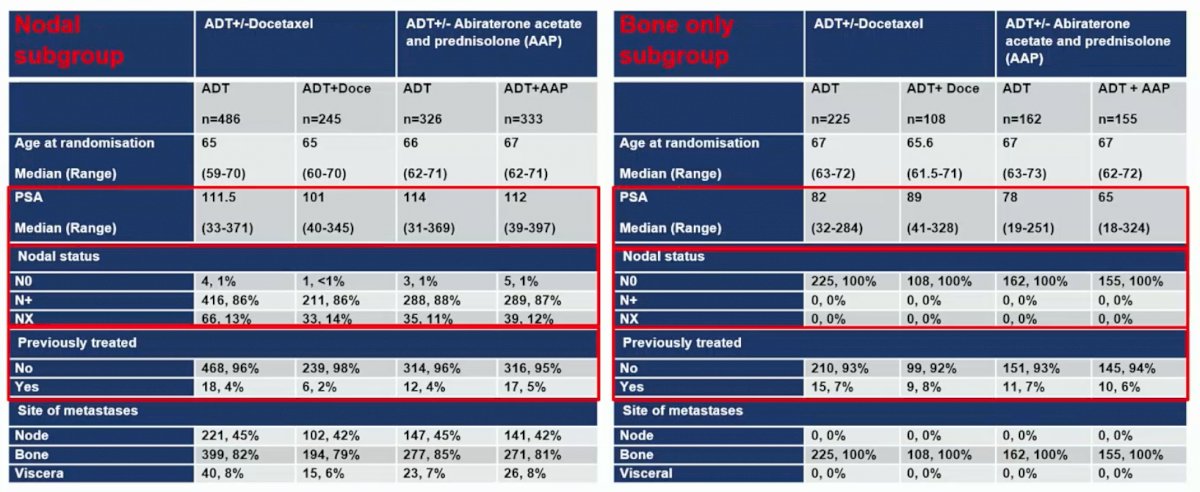
Baseline characteristics between those with nodal disease and bone-only disease showed a higher PSA among patients with lymph node disease:
Significant OS benefit was demonstrated with ADT + abiraterone acetate and prednisolone in both nodal +/- bone (HR 0.65, 95% CI 0.53-0.78) and bone-only metastases groups (HR 0.55, 95% CI 0.41-0.74). The interaction HR was 1.21, 95% CI 0.86-1.72 (p = 0.278):
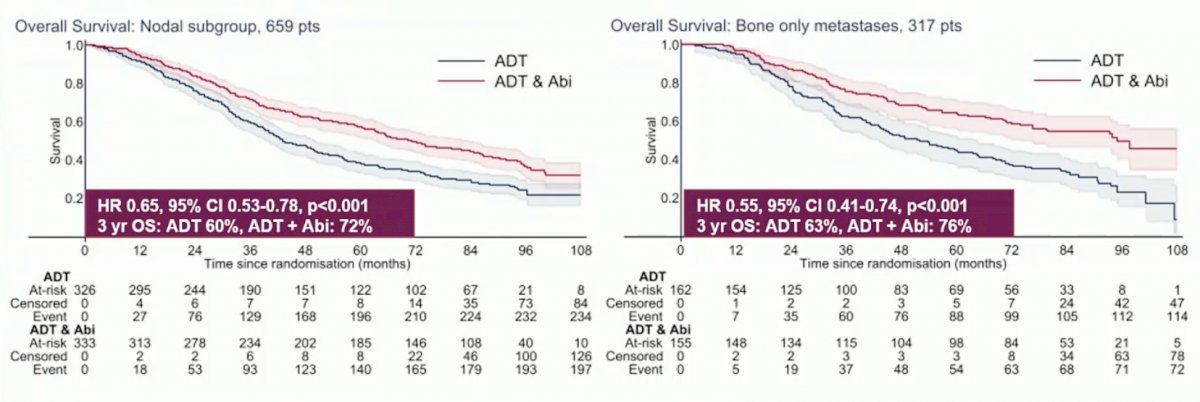
Patients with bone-only metastases treated with ADT + docetaxel had a similar survival benefit (HR 0.62, 95% CI 0.46-0.84). Notably, survival benefit was reduced for ADT + docetaxel in the nodal +/- bone metastases group (HR 0.89, 95% CI 0.74-1.07). This difference was statistically significant by test for interaction (HR 1.43, p = 0.046). Higher nodal burden had significantly worse outcomes in both control arms and the intervention arm of ADT +/- abiraterone acetate and prednisolone.
Dr. Haran concluded this presentation by discussing differential treatment response with nodal metastases in mHSPC and evaluation of nodal burden as a prognostic biomarker with the following take-home messages:
- Increased nodal burden is a negative prognostic biomarker and should be considered in prospective risk/volume definitions to aid risk stratification in selected patients
- This study also demonstrates for the first time a potential differential response between mHSPC patients with nodal +/-bone metastases versus bone-only metastases for ADT + docetaxel but not for ADT + abiraterone acetate and prednisolone
Presented by: Áine M. Haran, Genito-urinary Cancer Research Group, The University of Manchester, Manchester, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


