(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a presentation by Dr. Karim Fizazi discussing preliminary results of CYPIDES, a phase 2 trial of ODM-208 in mCRPC cancer patients. ODM-208 is a first in class, oral, non-steroidal, and selective inhibitor of CYP11A1, the first and rate-limiting enzyme of steroid biosynthesis:

ODM-208 suppresses the production of all steroid hormones and precursors that may activate the androgen receptor signaling pathway. This is particularly relevant in patients with androgen receptor ligand binding domain activating somatic point mutations, a mechanism of resistance to hormone-based therapies in mCRPC. At ESMO 2022, Dr. Fizazi and colleagues reported the first results of the phase 2 expansion of the CYPIDES trial.
ODM-208 5mg BID (with dexamethasone and fludrocortisone) was evaluated in an open-label phase 2 expansion cohort in patients with progressing mCRPC who had previously received ≥1 line of 2nd generation androgen receptor pathway inhibitor and ≥1 line of taxane-based chemotherapy. All patients had a pre-specified activating androgen receptor ligand binding domain mutation by pre-screening of cell-free DNA (Guardant 360). Study objectives were safety and preliminary efficacy assessed by PSA and RECIST response and standard safety measures. ODM-208 treatment was continued until subsequent disease progression. The study was conducted at 18 sites in France, Finland, UK and USA.
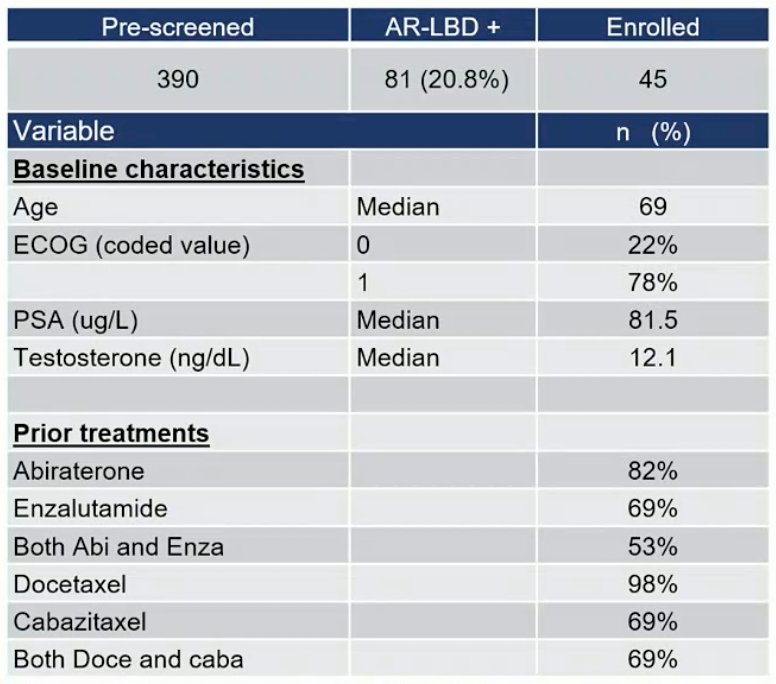
There were 81 of 390 (20.8%) pre-screened patients that had a pre-specified androgen receptor ligand binding domain mutation, of which 45 patients (median age 68 yrs) were enrolled and initiated ODM-208 treatment (43 at data cut-off of March 17, 2022). There were 53% of patients that had previously received both abiraterone and enzalutamide, and 69% patients received both docetaxel and cabazitaxel. The complete baseline characteristics are as follows:
Based on the emerging data ODM-208 profoundly suppressed androgen synthesis, resulting in >50% best PSA reduction 53% of patients:

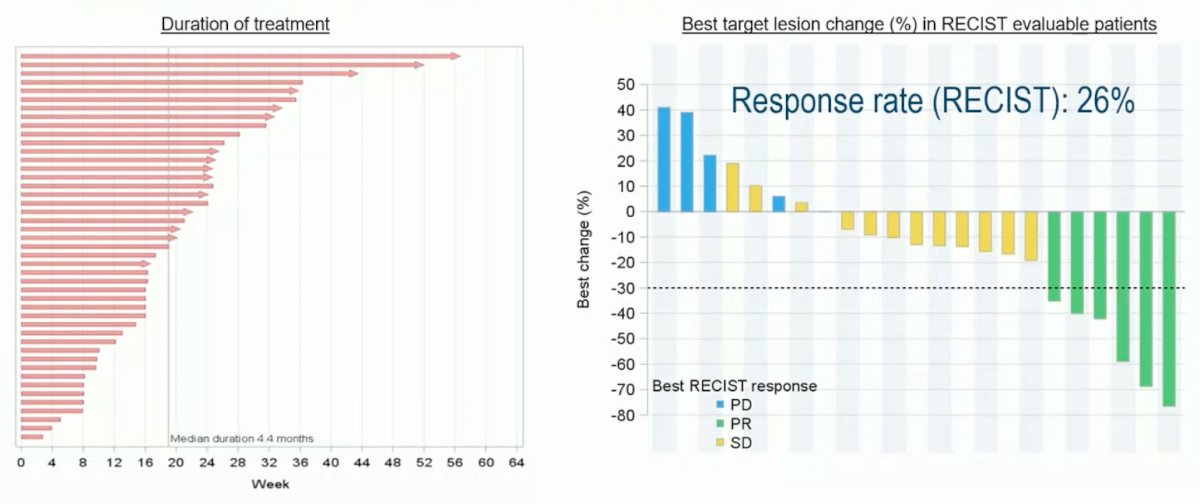
Furthermore, treatment duration is prolonged in many of these late-stage men; there were and at least four RECIST partial responses in 17 evaluable patients (an overall 26% response rate per RECIST):
ODM-208 has been well-tolerated with a much lower rate of hospitalization for adrenal insufficiency than in phase 1 with typically higher doses (2.3% vs. 33% to date). Grade >=3 adverse events occurred in 67% of patients, with the most common being anemia, fatigue, muscle spasm, tumor pain, insomnia, diarrhea, hyponatremia, and hyperkalemia. Overall, four patients withdrew due to adverse events, secondary to sepsis (n=1), hyperkalemia (n=1), acute cardiac insufficiency (n=1), and reduced general condition (n=1).
Dr. Fizazi concluded his presentation discussing preliminary results of CYPIDES, a phase 2 trial of ODM-208 in mCRPC cancer patients with the following concluding statements:
- In extensively pre-treated mCRPC patients, at a dose of ODM-208 (5mg BID):
- There was anti-cancer activity in mCRPC men with androgen receptor mutations
- The PSA50 response rate was >50%
- Could be safely given at 5 mg BID with adequate hormonal supplementation with few hospitalizations for adrenal insufficiency
- Some late-stage mCRPC men remain hormone dependent, despite prior ADT and androgen receptor inhibitors
- ODM-208 is a promising future treatment option
Presented by: Karim Fizazi, MD, Ph.D., is a medical oncologist at Gustave Roussy, and a full professor in Oncology at the University of Paris-Saclay in Villejuif, France.
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


