(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a presentation by Dr. Stephane Oudard discussing results of CABASTY, a randomized, phase III trial assessing cabazitaxel every 2 weeks versus every 3 weeks in older patients with mCRPC. Cabazitaxel 25 mg/m2 every 3 weeks (q3w) + G-CSF prolongs overall survival (OS) vs abiraterone or enzalutamide in mCRPC patients previously treated with docetaxel and the alternative androgen-targeted agent.1 Additionally, pilot studies suggest that cabazitaxel 16 mg/m2 every 2 weeks (q2w) induces less severe neutropenia and could be useful for older patients unfit to receive cabazitaxel q3w.2 The CABASTY trial aimed to confirm this hypothesis.
Patients enrolled in CABASTY had progressive mCRPC (≥65 years of age, ECOG performance status 0-2, G8 >14 or ≤14 with reversible geriatric impairment) and were previously treated with docetaxel. Patients were randomized to cabazitaxel q3w + prednisone + G-CSF vs cabazitaxel q2w + prednisone + G-CSF and stratified by age (<70 vs ≥ 70 yrs) and G8 (>14 vs ≤14). The primary endpoint was the percentage of patients with grade ≥3 neutropenia and/or neutropenic complications (febrile neutropenia, neutropenic infection, sepsis). Secondary endpoints were radiographic progression-free survival (rPFS), objective tumor response, skeletal related events (SREs), PSA response, quality of life (not reported at ESMO 2022), safety, and OS:
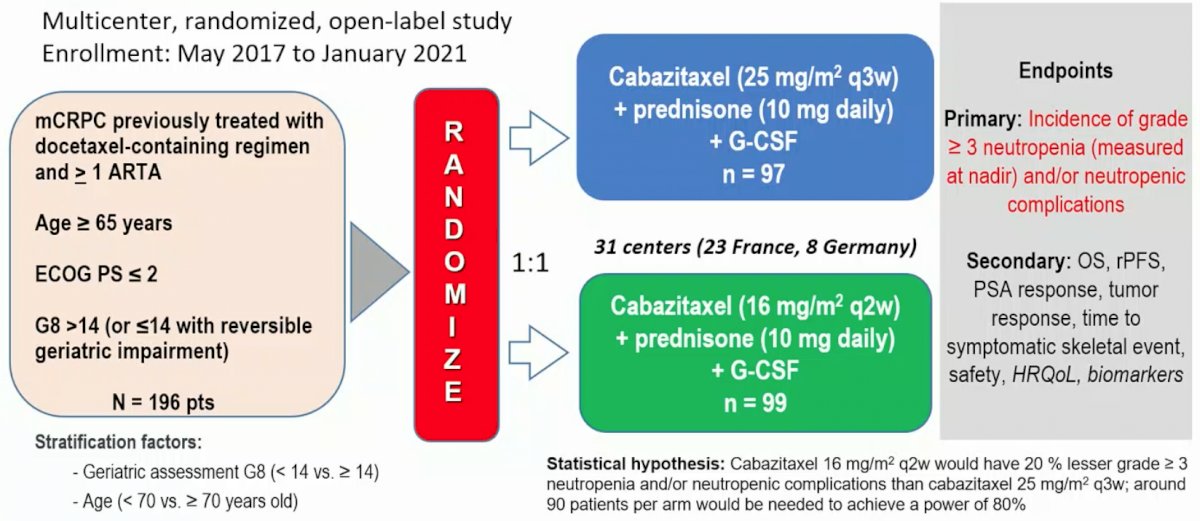
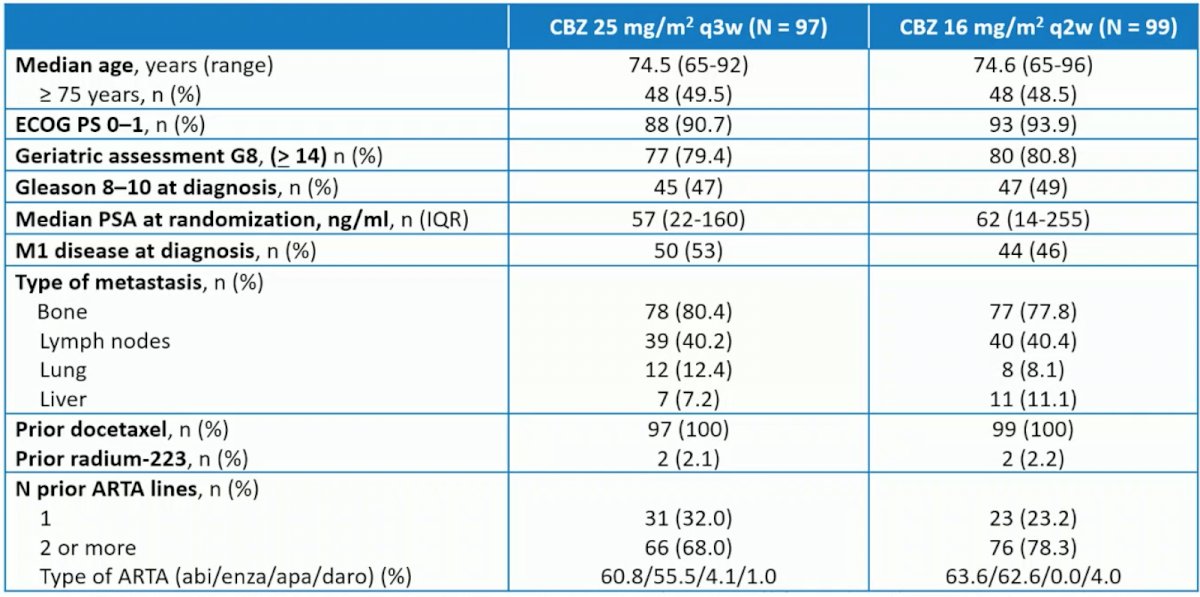
Overall, 196 patients (median age, 74.0 years, with 79.6% ≥ 70 years; 19.9% G8 <14; 30.1% vulnerable or frail per SIOG guidelines; 86.7% treated with prior androgen-targeted agent) were randomized to cabazitaxel q3w (n = 97) versus cabazitaxel q2w (n = 99). Baseline characteristics for the two groups is as follows:
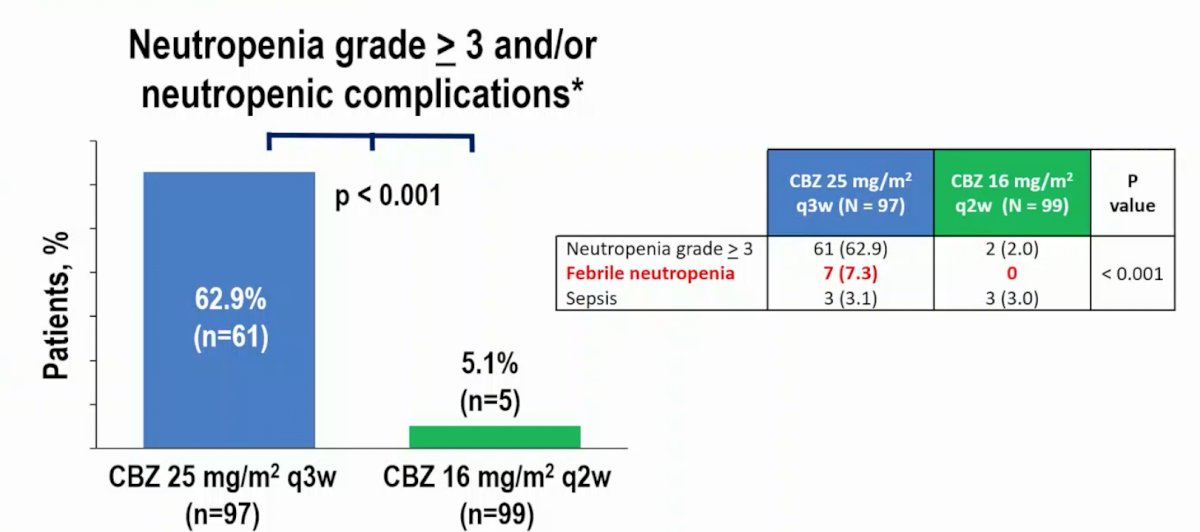
The rate of Grade ≥3 neutropenia and/or neutropenic complications was significantly higher with cabazitaxel q3w vs cabazitaxel q2w (62.9% vs 5.1%; OR 0.03, 95% CI 29.5-48.9, p<0.001):
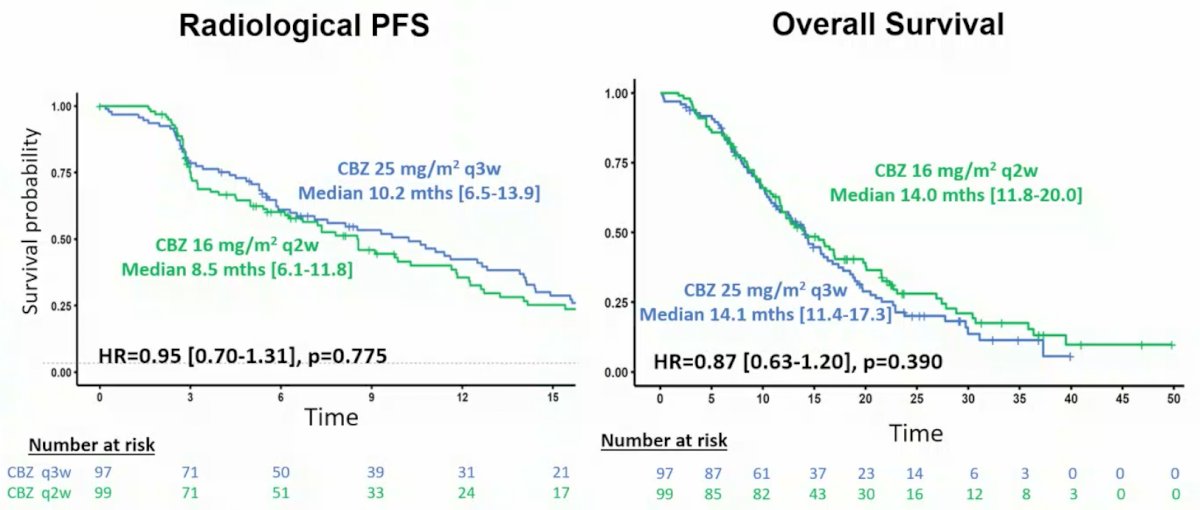
There was no difference in the secondary endpoints of radiological PFS or OS between the two groups:
Additionally, there was no difference in PSA response >=50% and objective tumor response:

Relative dose intensity for cabazitaxel q3w vs cabazitaxel q2w was comparable (92.3% vs 91.6%). Grade ≥3 adverse events were more common with cabazitaxel q3w (72.9% vs 58.2%). One patient (cabazitaxel q3w arm) died of neutropenic complications. There were no new safety signals observed.
Dr. Oudard concluded his presentation discussing the results of CABASTY, a randomized, phase III trial assessing cabazitaxel every 2 weeks versus every 3 weeks in older patients with mCRPC with the following take-home messages:
- The CABASTY trial met its primary endpoint – in this heavily pretreated elderly mCRPC population (50% >= 75 years), cabazitaxel 16 mg/m2 q2w plus G-CSF significantly reduced the rate of grade >= 3 neutropenia and/or neutropenic complications versus cabazitaxel 25 mg/m2 q3w plus G-CSF
- Secondary efficacy endpoints (OS, rPFS, PSA response, objective tumor response) were comparable between the two arms
- There were no new safety signals with cabazitaxel 16 mg/m2 q2w plus G-CSF
- Cabazitaxel 16 mg/m2 q2w plus G-CSF should be offered to older patients with mCRPC who are considered unfit for the standard cabazitaxel regimen
Presented by: Stephane Oudard, Oncology Department, HEGP - Hopital Europeen Georges-Pompidou - APHP - University de Paris, Paris, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
- Clement-Zhao A, Auvray M, Aboudagga H, et al. Safety and efficacy of 2-weekly cabazitaxel in metastatic castration-resistant prostate cancer. BJU Int. 2018 Feb;121(2):203-208.


