(UroToday.com) The 2022 ESMO annual meeting featured a prostate cancer session, including a discussant presentation by Dr. Martijn Lolkema discussing four abstracts presented at ESMO 2022 including “SAKK 08/14 - IMPROVE Investigation of metformin in patients with mCRPC in combination with enzalutamide versus enzalutamide alone: A randomized, open label, phase II trial” presented by Dr. Christian Rothermundt, “Pembrolizumab + olaparib versus abiraterone or enzalutamide for patients with previously treated mCRPC: randomized open-label phase 3 KEYLYNK-010 study” presented by Dr. Evan Yu, “Cabazitaxel every 2 weeks versus every 3 weeks in older patients with mCRPC: The CABASTY randomized phase III trial” presented by Dr. Stephane Oudard, and “Preliminary Phase 2 results of the CYPIDES study of ODM-208 in mCRPC cancer patients” presented by Dr. Karim Fizazi. Dr. Lolkema started by noting that there are several advances beyond/besides PSMA targeting in metastatic prostate cancer:

Both the SAKK 08/14 – IMPROVE (enzalutamide + metformin) and CYPIDES (ODM-208) trials implemented treatments that target the androgen receptor. Dr. Lolkema notes that the remaining opportunities in the androgen receptor targeting world include the following:

Dr. Lolkema emphasized that treatment with ODM-208 is akin to a ‘chemical adrenalectomy’ with a preference for mutants with ligand promiscuity. Interestingly, at the population level 6.4% of patients with CRPC have ligand binding domain mutations of the androgen receptor, however, in the Fizazi et al. study 20.8% of patients were positive for ligand-binding mutations. Thus, is this secondary to selection bias or underestimation in previous studies? The rationale to only include ligand binding domain mutated patients enriches for responders but is not completely targeted. ODM-208 in CYPIDES profoundly suppressed androgen synthesis, resulting in >50% best PSA reduction in 53% of patients, with treatment duration being prolonged in many of these late-stage men. Additionally, there was a 26% response rate per RECIST, and median duration of treatment of 4.4 months. At the dose tested, there were manageable but significant side effects, with 67% of patients having grade 3 or higher adverse events and 4/45 patients withdrawing from the study secondary to toxicity reasons. Dr. Lolkema also noted that a single arm design does not allow for direct comparisons.
Dr. Lolkema provided the following conclusions for the phase 2 CYPIDES study:
- If indeed 20% of patients were positive for ligand binding domain mutations, this treatment has a large patient pool (comparable to HRD deficiency) and is potentially effective
- The authors have to be applauded for selecting a population that could benefit and toxicity seems manageable
- However, comparison with dexamethasone alone is very important, and competition with PSMA targeted agents should be considered. Whether ODM-208 beats Lu-177-PSMA or other PSMA targeted strategies is unclear
- This is another step towards more personalized androgen receptor targeting (after AR-V7) and increases the need to standardize the predictive/prognostic testing and to perform more comprehensive testing
The SAKK 08/14 – IMPROVE trial was designed to assess the usefulness of metformin as an adjunct for enzalutamide in men with mCRPC. However, the primary endpoint for this trial was not met: disease control rate at 15 months was 52.4% for enzalutamide + metformin (90% CI 42.9% - 61.8%) and 56.1% for enzalutamide (90% CI 46.4% - 65.4%), respectively (p = 0.644). Based on an unplanned subgroup analysis, Dr. Lolkema wonders if overweight may potentially be a biomarker.
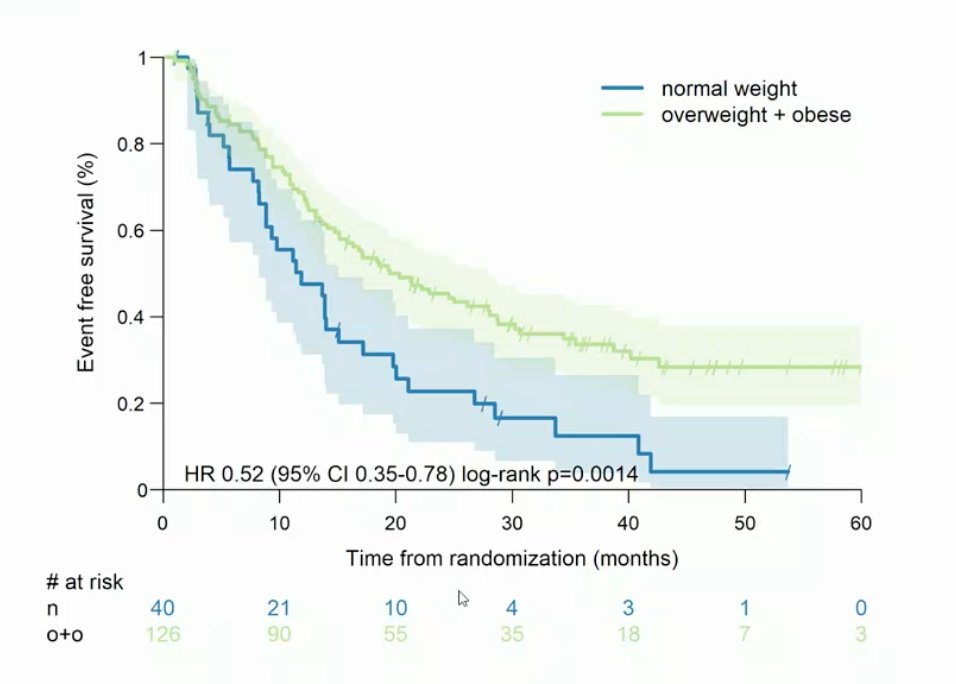
Additionally, Dr. Lolkema emphasized that to improve patient selection for this strategy, PTEN loss patients should have probably been excluded from this trial to optimize results. Dr. Lolkema provided the following conclusions for the SAKK 08/14 – IMPROVE trial:
- Metformin does not improve the activity of enzalutamide, despite the strong pre-clinical rationale
- This investigator driven study shows that it is possible to do impactful academic trials and this needs to be recognized
- However, the patient selection could have been improved, as PTEN loss patients should have been excluded
- Exploratory analyses only showed a trend towards advantage in time to PSA progression, thus there may be a signal, but it is not very strong
- Co-inhibition of the PI3K pathway may be effective in selected patient populations but needs better testing/selection strategies
Both the CABASTY (cabazitaxel) and KEYLYNK-010 (pembrolizumab + olaparib) trials implemented treatments that target the cell cycle and DNA repair deficiency. Patients enrolled in CABASTY had progressive mCRPC (≥65 years of age, ECOG performance status 0-2, G8 >14 or ≤14 with reversible geriatric impairment) and were previously treated with docetaxel. Patients were randomized to cabazitaxel q3w + prednisone + G-CSF vs cabazitaxel q2w + prednisone + G-CSF and stratified by age (<70 vs ≥ 70 yrs) and G8 (>14 vs ≤14). The rate of Grade ≥3 neutropenia and/or neutropenic complications was significantly higher with cabazitaxel q3w vs cabazitaxel q2w (62.9% vs 5.1%; OR 0.03, 95% CI 29.5-48.9, p<0.001):

Dr. Lolkema notes that when comparing the experimental arm of CABASTY to PROSELICA1 and TROPIC2 (which had younger patients than CABASTY), PSA50 response, OS, and grade >=3 neutropenia was comparable between these trials:
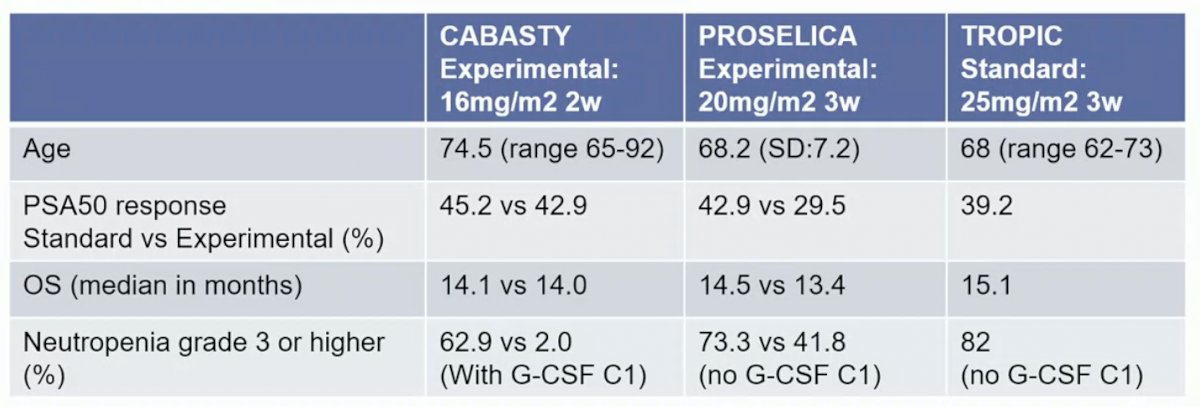
Even with older patients, results were similar and thus we should not exclude elderly patients from cabazitaxel treatment. Dr. Lolkema notes that there was no data on screen failure rate based on G8 assessment, thus we do not know for certain if this was truly a vulnerable population. However, all patients with a high G8 score were seen by a geriatrician, with the main reason for a bad score being: anemia (n = 14), asthenia (n = 13), high blood pressure (n = 10), and bone/back pain (n = 22). Dr. Lolkema provided the following conclusions for the CABASTY trial:
- Biweekly cabazitaxel maintains dose intensity of treatment with fewer grade 3 neutropenia events
- Elderly patients should thus be considered for cabazitaxel chemotherapy
- However, cabazitaxel 20 mg/m2 every 3 weeks is an equally effective and mild regimen
- The population seems well represented, but there is question as to how to deal with more vulnerable patients. G8 could be used to screen patients for geriatric referral
- The question is whether G-CSF prophylaxis is cost-effective with lower toxicity
- We now have a number of well-tolerated cabazitaxel treatment options and this should be an option for all prostate cancer patients and should be a control arm for more studies
KEYLYNK-010 is a phase 3 trial of patients that had mCRPC and progressed on or after abiraterone or enzalutamide (but not both) as well as docetaxel, and had ECOG performance status ≤1. Patients were randomized 2:1 to 200 mg pembrolizumab IV Q3W for ≤35 cycles + 300 mg olaparib orally BID, or to next-generation hormonal agent: 1000 mg abiraterone orally QD (if prior enzalutamide) or 160 mg enzalutamide orally QD (if prior abiraterone). The primary endpoints of rPFS (median 4.4 months with pembrolizumab + olaparib vs 4.2 months with next-generation hormonal agent; HR 1.02, 95% CI 0.82−1.25; p = 0.55) and OS (15.8 months vs 14.6 months; HR 0.94, 95% CI 0.77−1.14; p = 0.26) was not met:
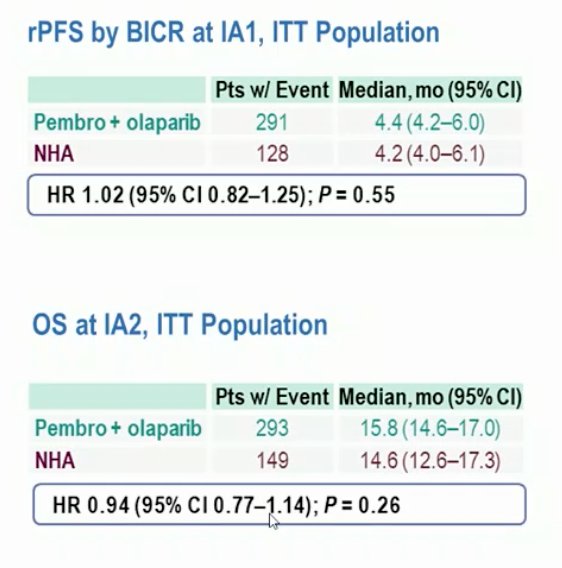
Dr. Lolkema emphasized that pembrolizumab in prostate cancer has had a poor start and that outside of the MSI-high subgroup there has not been a lot of activity. Olaparib shows promising activity in HRD patients and in combination with abiraterone + prednisone in the all comer population. However, the pre-clinical hypothesis is relatively poorly supported by data. Dr. Lolkema provided the following conclusions for the KEYLYNK-010 trial:
- Combining olaparib and pembrolizumab is an unselected patient population of metastatic prostate cancer is not effective
- The late stage setting after novel hormonal agent and chemotherapy may not be the best setting to test new therapeutic strategies
- Although switching novel hormonal agents is a common treatment strategy, we need to consider it as a control arm. Cabazitaxel and now Lu-177-PSMA should be considered as comparators
- More targeted and personalized approaches should be the way of the future. This trial is another example of why patient selection is very important
Presented by: Martijn P. Lolkema, MD, Erasmus University Medical Center, Rotterdam, The Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- Eisenberger M, Hardy-Bessard AC, Kim CS, et al. Phase III study comparing a reduced dose of cabazitaxel (20 mg/m2) and the currently approved dose (25 mg/m2) in postdocetaxel patients with metastatic castration-resistant prostate cancer—PROSELICA. J Clin Oncol 2017 Oct 1;35(28):3198-3206.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010;376(9747):1147-1154.


