(UroToday.com) During the Mini Oral session of the European Society for Medical Oncology (ESMO) Annual Congress focusing on non-prostate genitourinary cancers, Dr. Jad Chahoud presented a real-world analysis of neoadjuvant platinum-based chemotherapy (NAPC) in locally advanced squamous cell carcinoma of the penis. A benefit of this treatment approach has been previous suggested by prior small case series. Currently, this approach is being assessed in the phase III randomized InPACT trial. However, those data are not going to be available for at least 4 years. Thus, there is an ongoing evidence void.
These authors pooled data from eight tertiary care centers to identified patients who had undergone NAPC prior to surgical resection for locally advanced squamous cell carcinoma of the penis were analyzed. All included patients had locally advanced (cTany, cN+) disease. The authors collected data on clinicopathologic characteristics, agents utilized, and surgical modalities. They assessed the primary outcome of overall survival (OS) using the Kaplan-Meier Method and Cox Proportional Hazard modeling. They further examined the secondary outcome of best overall response measured with RECIST 1.1 criteria.
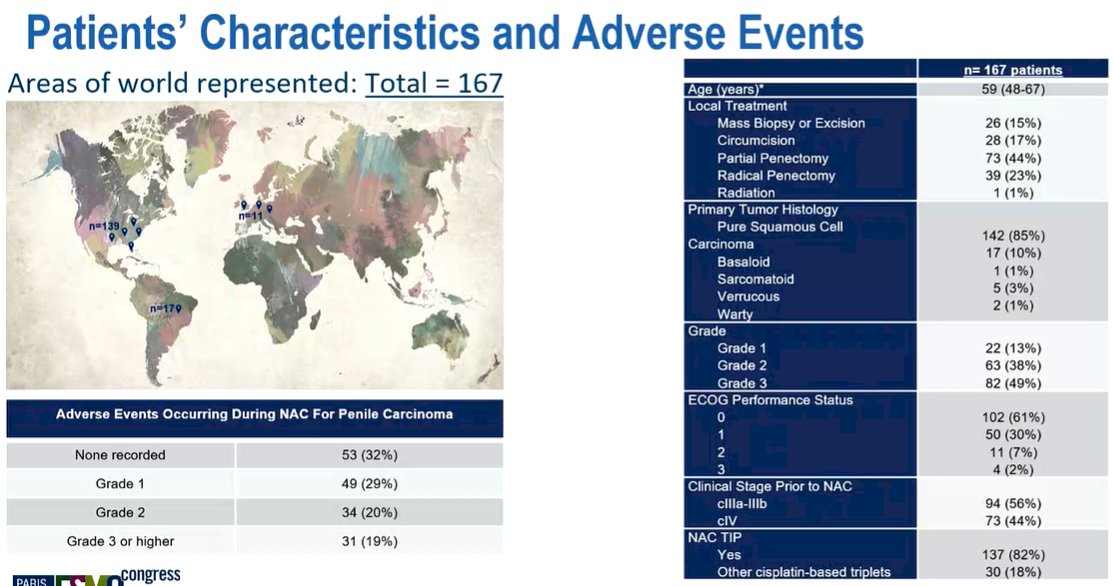
They included 156 patients treated with NAPC for locally advanced squamous cell carcinoma of the penis. The median age of the cohort was 59 years (range, 48-67), and 109 (70%) had an Eastern Cooperative Oncology Group score of 0-1. The clinical stages prior to NAPC were cIIA-IIB (12%), cIIIa (16%), cIIIb (22%), and cIV (47%). 123 (79%) of patients received TIP (Paclitaxel - Ifosfamide - Cisplatin) NAC prior to surgical resection. 76% of patients underwent penile-sparing surgical procedures, 24% had a radical penectomy and 84% had inguinal lymphadenectomy.
Assessing the primary outcome of overall survival, the authors found a median overall survival of 39 months (95% CI 20.9 – 57.1).
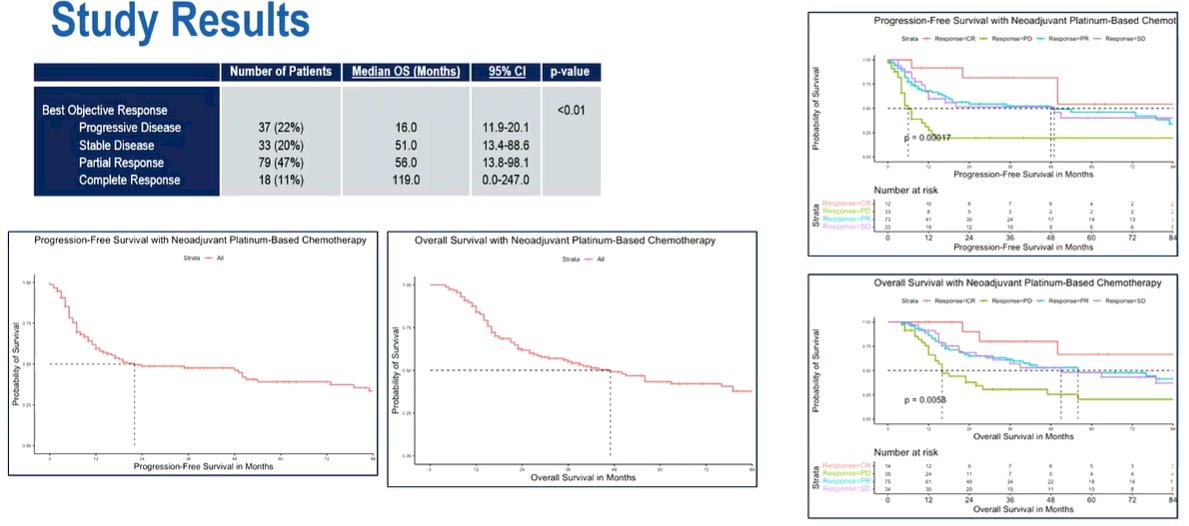
The median progression free survival was 23 months (95% CI 10.4 – 35.5). On serial imaging after NAPC, 20.7% of patients had progressive disease (PD), 23.3% had stable disease, 48.4% had partial response, and 7.6% had complete response.
Using Cox Regression modeling to assess significant predictors of overall survival, patients with progressive disease following administration of NAPC had an increased risk of mortality (HR 2.1, p=0.02). Patients who had evidence of downstaging following NAPC had improved overall survival. In contrast, as expected, those with residual ENE had a worse prognosis than those without.
Thus, Dr. Chahoud noted that NAPC is efficacious in locally advanced squamous cell carcinoma of the penis based on a large, multi-institutional analysis.
Presented by: Jad Chahoud, MD, MPH, Moffitt Cancer Center, Tampa, FloridaWritten by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.


