(UroToday.com) The 2023 ESMO annual meeting included a session on urothelial carcinoma, featuring a presentation by Dr. Bradley McGregor discussing the Double Antibody Drug (DAD) conjugate phase I trial of sacituzumab govitecan plus enfortumab vedotin as ≥ second line therapy for metastatic urothelial carcinoma. Over the last several years, antibody-drug conjugates have become a mainstay in treatment of metastatic urothelial carcinoma. Sacituzumab govitecan and enfortumab vedotin are antibody drug conjugates used sequentially in the management of treatment resistance metastatic urothelial carcinoma. Given different targets, payloads with non-overlapping toxicities, and preclinical synergy of sacituzumab govitecan with anti-microtubule agents, Dr. McGregor and colleagues evaluated the safety and efficacy of sacituzumab govitecan + enfortumab vedotin in a phase I trial.
Patients with metastatic urothelial carcinoma and ECOG ≤1 who progressed on platinum and immunotherapy or were cisplatin ineligible and received one line of therapy were enrolled. Key exclusion criteria included small cell carcinoma and uncontrolled diabetes. Sacituzumab govitecan + enfortumab vedotin were dosed D1, 8 of a 21-day cycle until progression of unacceptable toxicity. To assess the feasibility and safety of combining sacituzumab govitecan + enfortumab vedotin, doses were adjusted based on the incidence of dose-limiting toxicities during cycle 1 and the total number of patients treated at four pre-specified dose levels using a Bayesian Optimal Interval design: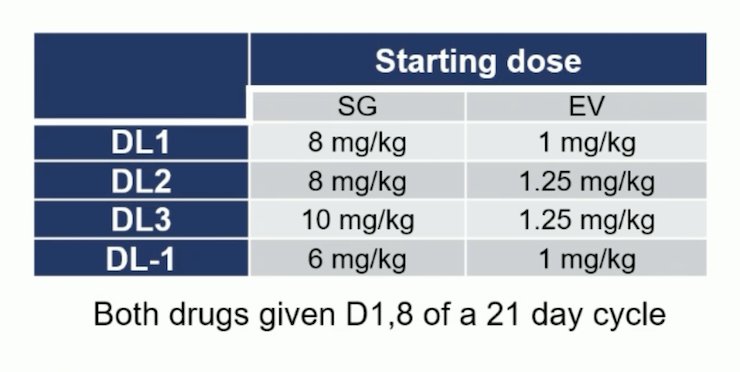
There were 24 patients enrolled (9 DL1, 9 DL2, 6 DL3) from May 2021 to April 2023. At the data cutoff of May 1, 2023, 23 patients were evaluable for dose-limiting toxicity assessment; one patient in DL3 never started therapy. The median age was 70 years (range 41-88) and 22 received ≥ 2 lines of therapy. The complete baseline characteristics are as follows:
After two patients in DL1 experienced febrile neutropenia, prophylactic granulocyte stimulating factor (GCSF) was permitted, among which 18 patients received GCSF. The data for maximum tolerated dose are as follows: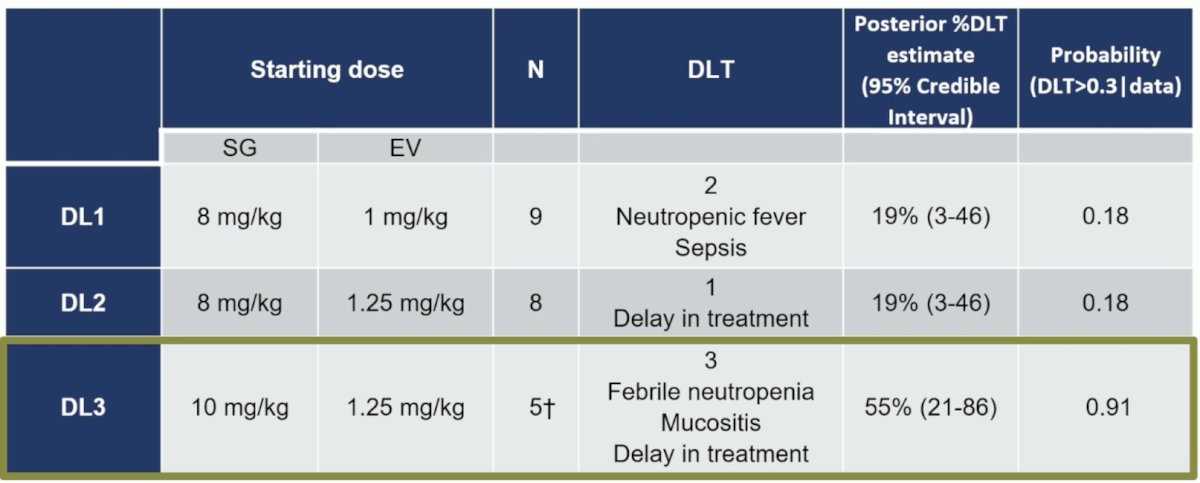
There were 70% of patients that experienced ≥ grade 3 adverse events at any dose levels with one grade 5 adverse events (pneumonitis possibly related to enfortumab vedotin) during C2 (patient in DL3): 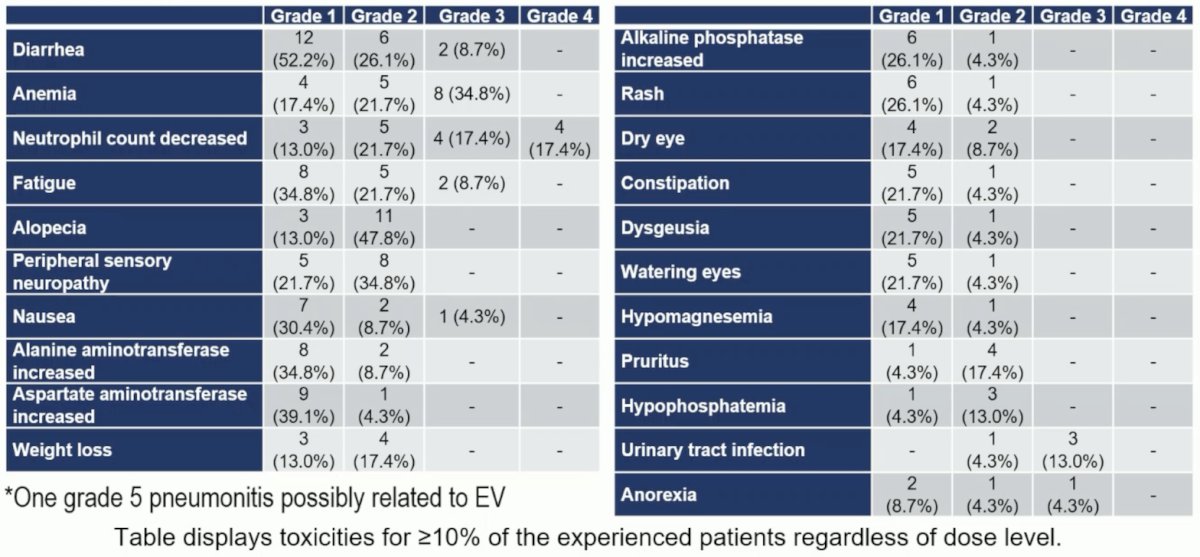
The recommended phase 2 dose was DL2: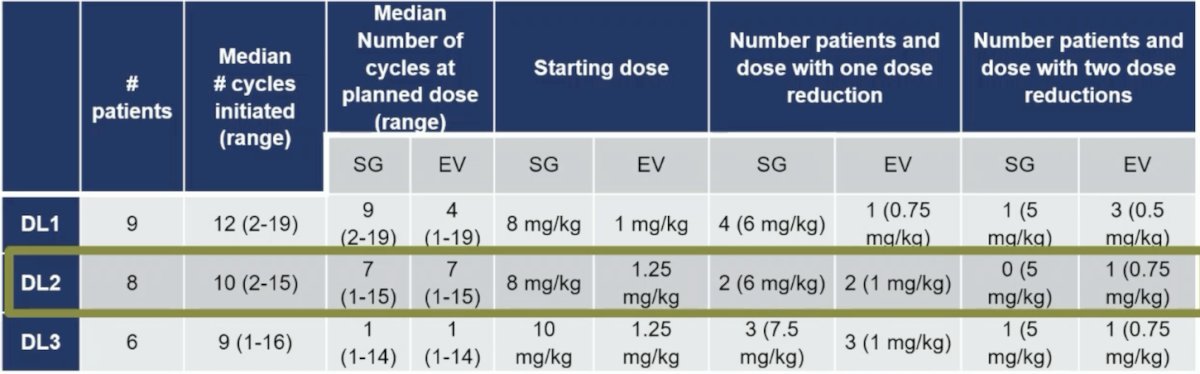
Overall, 20 of 23 patients had some degree of tumor shrinkage of their target lesion:
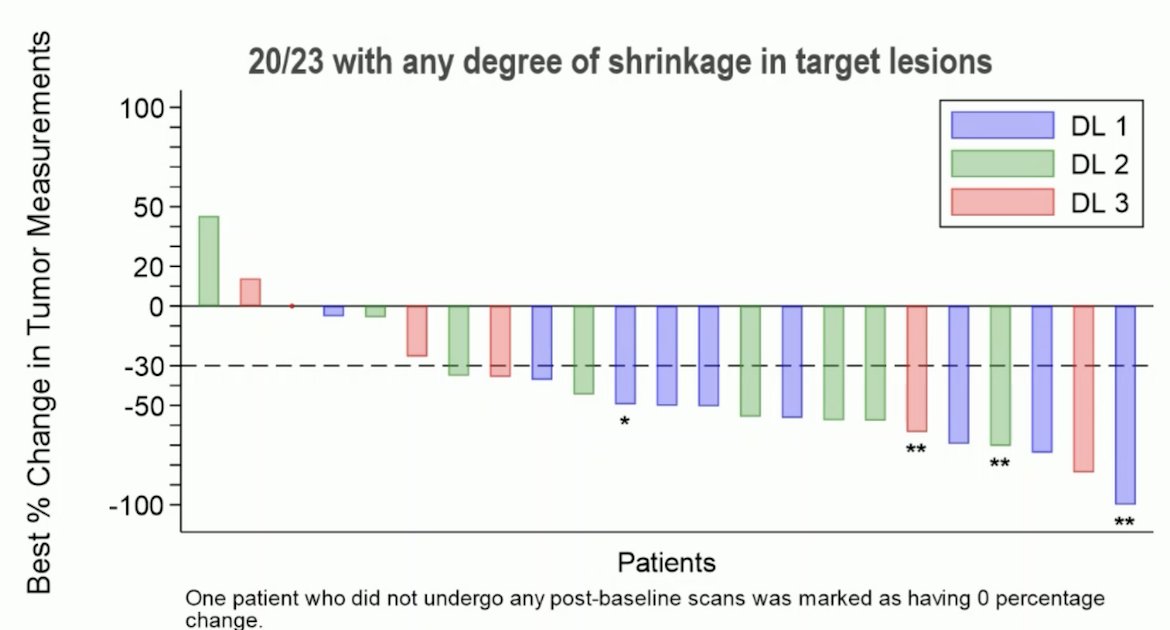
Objective response rate was 70% (15/21, 95% CI 47-87) with three complete responses, 13 partial responses, and three patients with progressive disease. The 12 month PFS rate was 41% (95% CI 18-62) and the 12 month OS rate was 86% (95% CI 61-95).
Dr. McGregor concluded his presentation discussing the DAD conjugate phase I trial of sacituzumab govitecan plus enfortumab vedotin as ≥ second line therapy for metastatic urothelial carcinoma with the following take-home points:
- DAD is the first trial (in any malignancy) to show antibody drug conjugates can be safely given in combination
- There were no new safety signals compared to monotherapy
- Combination maximal tolerated doses were sacituzumab govitecan 10 mg/kg + enfortumab vedotin 1.25 mg/kg D1, 8 every 21 days
- Accounting for cumulative toxicities, the recommended phase 2 dose for combination is sacituzumab govitecan 8 mg/kg + enfortumab vedotin 1.25 mg/kg D1, 8 every 21 days with GCSF support
- The objective response rate was 70% (95% CI 47-87%) and 9/23 patients have ongoing responses with a median 15 month follow-up
- Further studies are in development exploring sacituzumab govitecan and enfortumab vedotin alone or with pembrolizumab (DAD-IO trial) in urothelial carcinoma
Dr. McGregor noted that this trial was concomitantly published in Annals of Oncology.1
Presented by: Bradley A. McGregor, MD, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- McGregor BA, Sonpavde GP, Kwak L, et al. The Double Antibody Drug conjugate (DAD) phase 1 trial: Sacituzumab govitecan plus enfortumab vedotin for metastatic urothelial carcinoma. Ann Oncol 2023 Oct 21 [Epub ahead of print].


