The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a urothelial and other cancers abstract poster session for trials in progress. Dr. Andrea Necchi presented the study framework for SunRISe-3, a phase 3 randomized trial of TAR-200 plus cetrelimab or TAR-200 monotherapy versus intravesical BCG in patients with BCG-naïve, high-risk, non-muscle invasive bladder cancer (NMIBC).
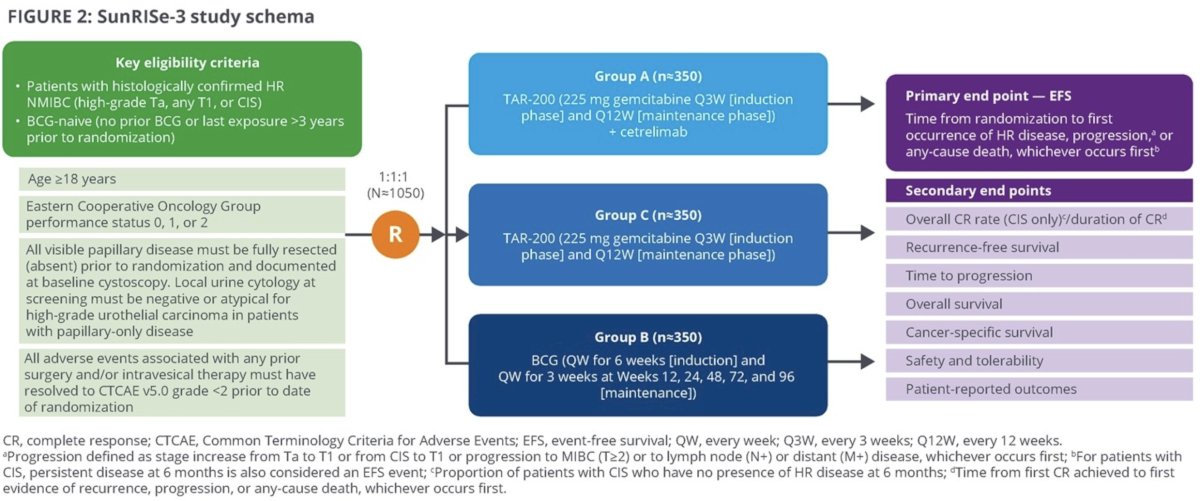
The current standard of care for patients with high-risk NMIBC remains a TURBT followed by intravesical BCG. However, we have recently experienced a global shortage of BCG, limiting clinical availability/utilization. Furthermore, BCG may be associated with significant adverse events and lacks a durable response in a significant proportion of such patients (i.e., BCG unresponsive). TAR-200 is an intravesical drug delivery system that provides local continuous gemcitabine release within the bladder. Preliminary data from SunRISe-1 were recently presented at AUA 2023 and demonstrated that 73% of patients with BCG-unresponsive NMIBC experience a complete response with TAR-200 monotherapy (38% for cetrelimab monotherapy). Dr. Necchi argued that this data supports the additional investigation of TAR-200 +/- cetrelimab in patients with BCG-naïve, high-risk NMIBC. SunRISe-3 (NCT05714202) is an open-label, multicenter, randomized phase 3 study evaluating the efficacy and safety of TAR-200 + cetrelimab or TAR-200 monotherapy versus BCG in patients with BCG-naïve, high-risk NMIBC.

This trial will include patients meeting the following eligibility criteria:
- Age ≥18 years
- ECOG performance status 0 – 2
- Histologically confirmed high-risk NMIBC (HG Ta, any T1 or CIS)
- BCG-naïve (no prior BCG or stopped >3 years prior to randomization)
- All papillary disease fully resected prior to randomization
A projected 1,050 patients will be randomized 1:1:1 as follows:
- Group A: TAR-200 + cetrelimab
- Group B: BCG
- Group C: TAR-200
In Groups A and C, TAR-200 (225 mg gemcitabine) will be delivered every 3 weeks during an induction phase, then every 12 weeks during a maintenance phase. In Group B, BCG will be instilled every week for 6 weeks (induction) followed by weekly for 3 weeks at weeks 12, 24, 48, 72, and 96. Treatment may be continued in year 3 at the investigator’s discretion.
Disease surveillance will be performed via cystoscopy, urine cytology, TURBT/bladder biopsy, and CT/MR urography. The primary endpoint is event-free survival, defined as time from randomization to the 1st recurrence of high-risk disease (HG Ta, any T1, or CIS), disease progression, any cause death, or persistent disease at 6 months in CIS patients. Progression is defined as a stage worsening (Ta to T1 or CIS to T1) or development of muscle-invasive disease (≥cT2 or cN+/M+). Secondary endpoints include:
- Overall complete response rate in CIS patients
- Recurrence-free survival
- Time to disease progression
- Overall survival
- Cancer-specific survival
- Safety
- Patient reported outcomes
As of May 3, 2023, 1 patient has been randomized, with 16 in screening; recruitment is planned at 254 sites.

Presented by: Andrea Necchi, MD, Professor, Director of GU Medical Oncology, San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


