(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a urothelial cancer abstracts poster session. Dr. Amanda Nizam presented the results of UNITE, a study evaluating biomarkers of treatment-related toxicity in advanced urothelial carcinoma patients treated with enfortumab vedotin (EV).
EV is a Nectin-4-directed antibody and microtubule inhibitor conjugate indicated as monotherapy for the treatment of patients with metastatic urothelial carcinoma who have previously received both a PD-1/L1 inhibitor and platinum-based chemotherapy or are ineligible for cisplatin-containing chemotherapy and have received ≥ 1 prior lines of therapy (July 2021). This is based on the results of EV-301which was a global, open-label phase 3 trial that demonstrated that EV, compared to investigator-chosen chemotherapy (standard docetaxel, paclitaxel, or vinflunine), was associated with overall survival benefits in this setting (median OS: 13 versus 9 months; HR: 0.70, 95% CI: 0.56 to 0.89, p=0.001).1
The incidence of any grade and grade 3 or worse treatment-related adverse events were similar in the two arms (93.9% and 51.4%, respectively for EV).1 As such, treatment duration is often limited by toxicity. The objective of UNITE was to evaluate patient characteristics and biomarkers associated with EV-related adverse events.
UNITE is a multicenter, retrospective study of EV monotherapy-treated advanced urothelial carcinoma patients who had available adverse events and next-generation sequencing (NGS) data. EV adverse events were assessed per CTCAE v5.0 criteria. The univariable associations between clinical features or somatic gene alterations (based on tissue or ctDNA) occurring in ≥5% of patients and the most common EV adverse events and treatment modifications (dose reduction, hold, or discontinuation) were assessed using the Wilcoxon rank sum and Chi-squared tests. Using Cox regression models, gene alterations were individually evaluated in separate multivariable) models, adjusting for relevant clinical factors.
This analysis included 607 patients from 16 US sites, of whom 275 had available EV adverse event and NGS data. At EV initiation, the median patient age was 71 years, 67% were men, 84% were White, 79% had ECOG performance status of 0 – 1, and 68% had received ≥2 prior lines of therapy.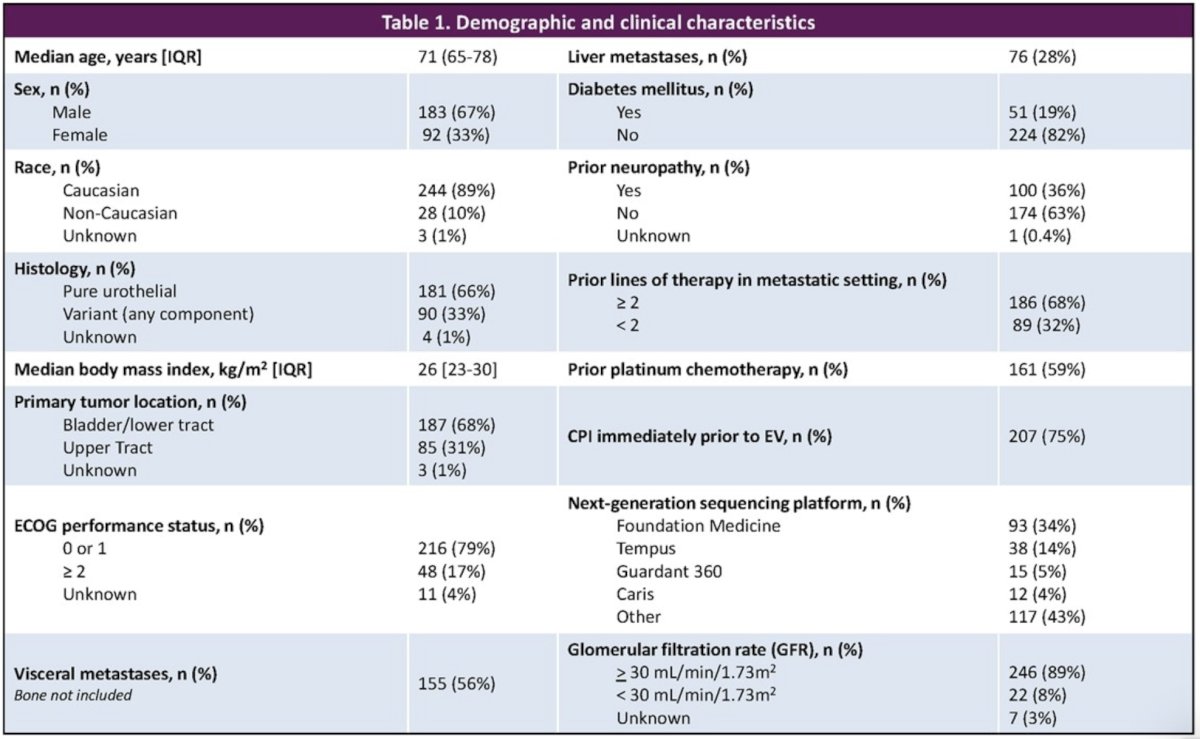
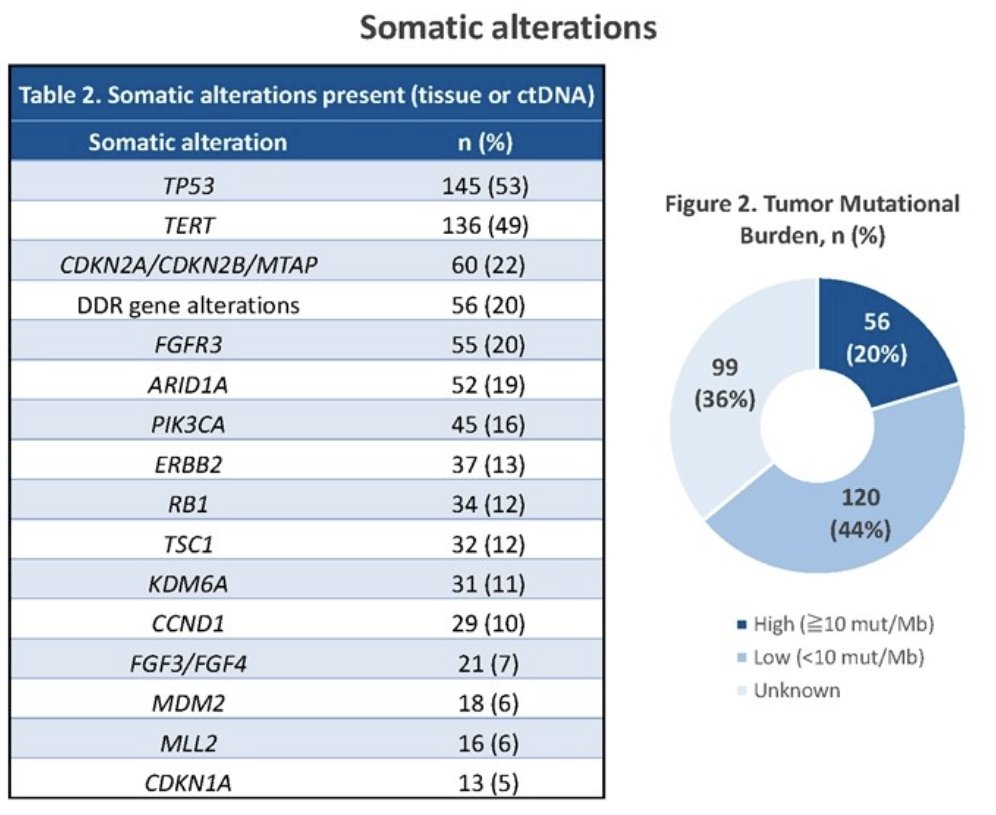
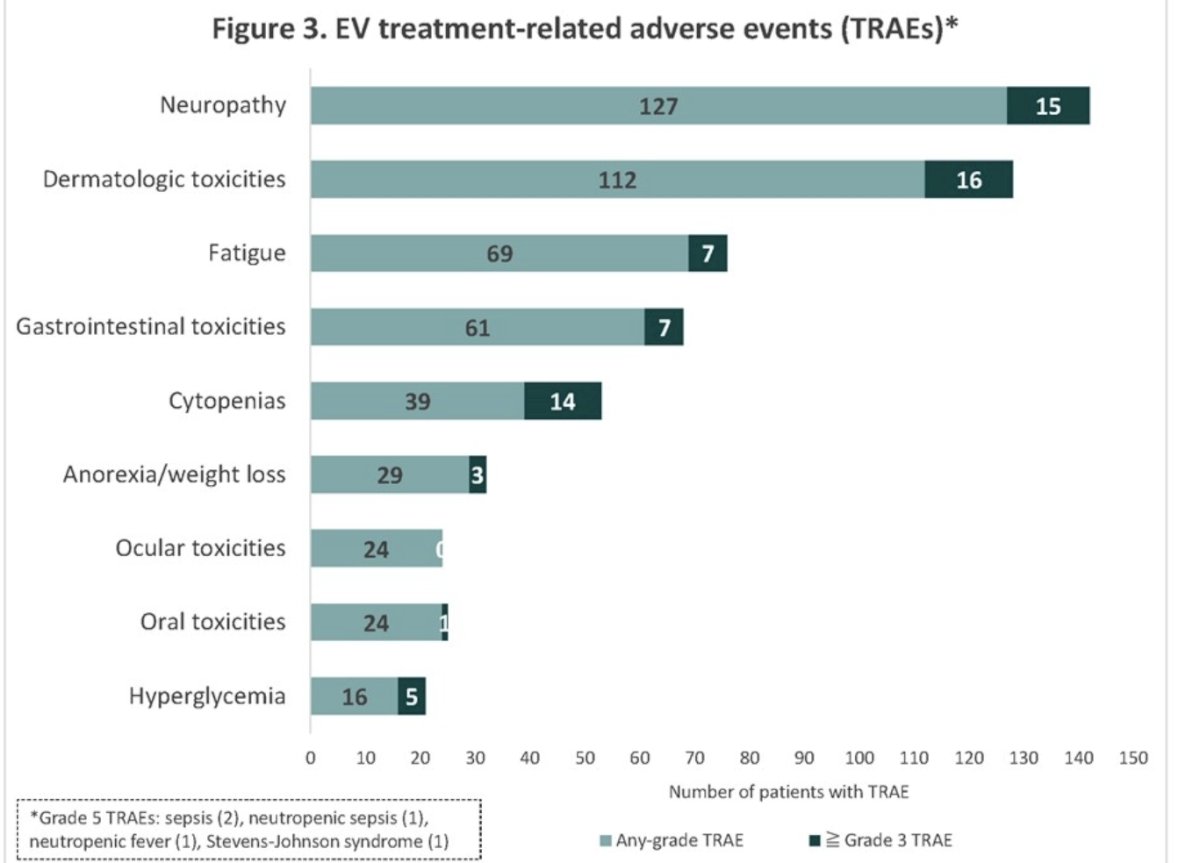
The median time from the initial diagnosis to EV start was 86 weeks (IQR: 48 to 165). Notably, the median time from EV start to adverse event onset was 4 weeks (IQR: 2 – 11). The most common all-grade adverse events were:
- Neuropathy (46%)
- Skin toxicity (41%)
- Fatigue (25%)
- GI toxicity (22%)
- Cytopenias (14%)
- Anorexia (11%)
On univariable analysis, significant predictors of neuropathy included diabetes, prior neuropathy, and higher hemoglobin and albumin at EV initiation (p for all <0.005). The predictors for skin toxicity included a lower white blood cell count, lower neutrophil: lymphocyte ratio, and GFR ≥30 ml/min (p for all <0.01). Predictors of cytopenias included a TERT gene alteration and a lower absolute lymphocyte count (p<0.01). Anorexia was significantly more common among those who were male (p=0.03), had liver metastases (p=0.05), and had a KDM6A gene alteration (p=0.04). Hyperglycemia was associated with a higher BMI, diabetes mellitus, lower neutrophil: lymphocyte ratio, and male sex (p for all <0.01). Higher BMI and lower neutrophil: lymphocyte ratio were significantly associated with more frequent treatment modifications in 203 pts (p<0.05).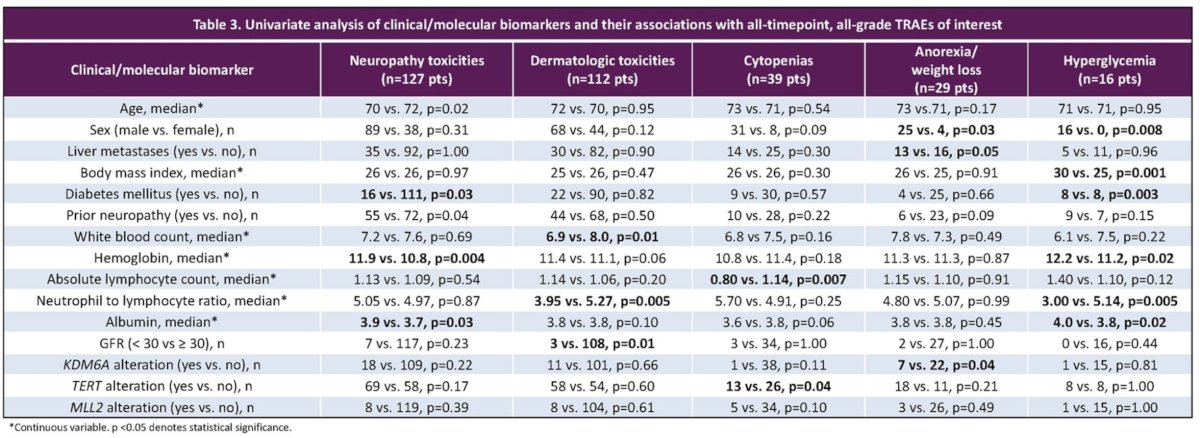
On multivariable analysis, MLL2 gene alteration was associated with cytopenias (p=0.05) and KDM6A with anorexia (p=0.02).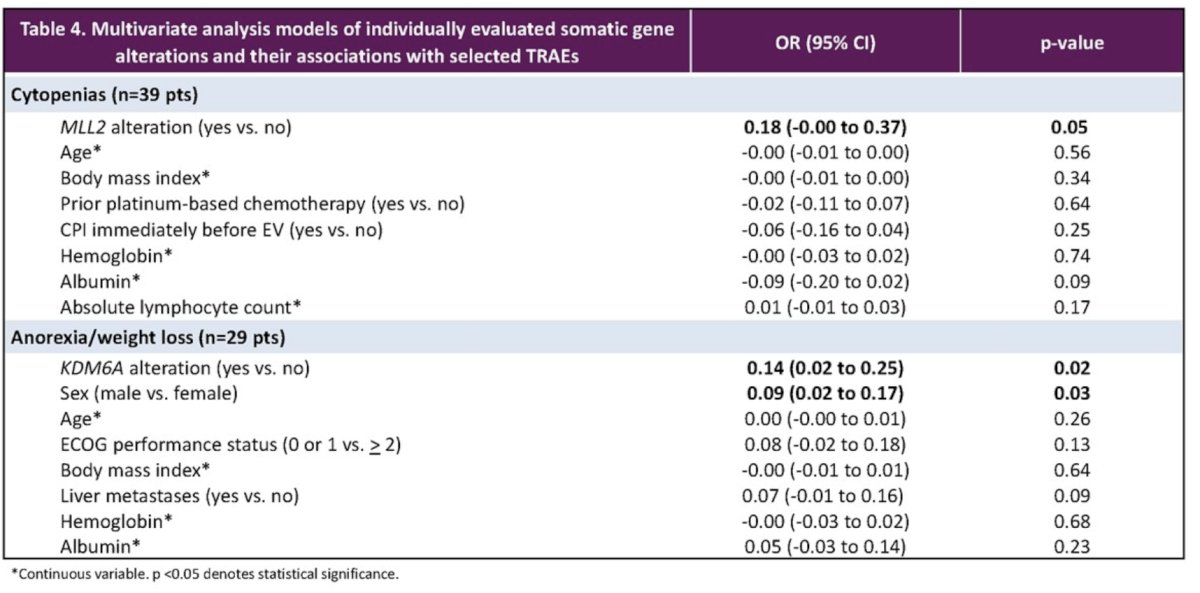
Dr. Nizam concluded that in EV-treated urothelial carcinoma patients, clinical variables and certain somatic gene alterations were associated with common EV adverse events. These hypothesis-generating findings suggest a limited role of somatic gene alterations in predicting EV adverse events, warranting further investigation of host and tumor-related factors underlying EV toxicity.
Presented by: Amanda, Nizam, MD, Hematology and Medical Oncology, Cleveland Clinic, Cleveland, OH
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
Reference:- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.


