(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Yohann Loriot presented the results of subgroup analyses from the phase III THOR study of erdafitinib versus chemotherapy in patients with advanced or metastatic urothelial carcinoma with select Fibroblast Growth Factor Receptor (FGFR) alterations.
There remains an unmet need for post-checkpoint inhibitor (CPI) therapies in the metastatic urothelial carcinoma population. Checkpoint inhibitors are currently recommended in both the 1st and 2nd line treatment settings; however, treatment options following progression on PD-(L)1 inhibitors are limited. It is currently estimated that 21% of patients with metastatic urothelial carcinoma respond to immune CPIs.1 Furthermore, in the real-world setting, only 30% of patients receive a subsequent anti-cancer treatment following anti-PD-(L)1 discontinuation.2
FGFR alterations are observed in ~20% of advanced or metastatic urothelial carcinoma patients, with this proportion even higher in those with metastatic upper tract urothelial carcinoma (35%), and these alterations may function as oncogenic drivers. Erdafitinib is an oral selective pan-FGFR tyrosine kinase inhibitor (TKI) that was granted accelerated approval in the United States and is approved in 18 other countries to treat locally advanced or metastatic urothelial carcinoma in adults with susceptible FGFR3/2alt with disease progression during or after platinum-containing chemotherapy. In the single arm phase 2 BLC2001 trial, erdafitinib demonstrated efficacy in patients with FGFR-altered advanced urothelial cancer:
- ORR: 40%
- Median PFS: 5.5 months
- Median OS: 11.3 months
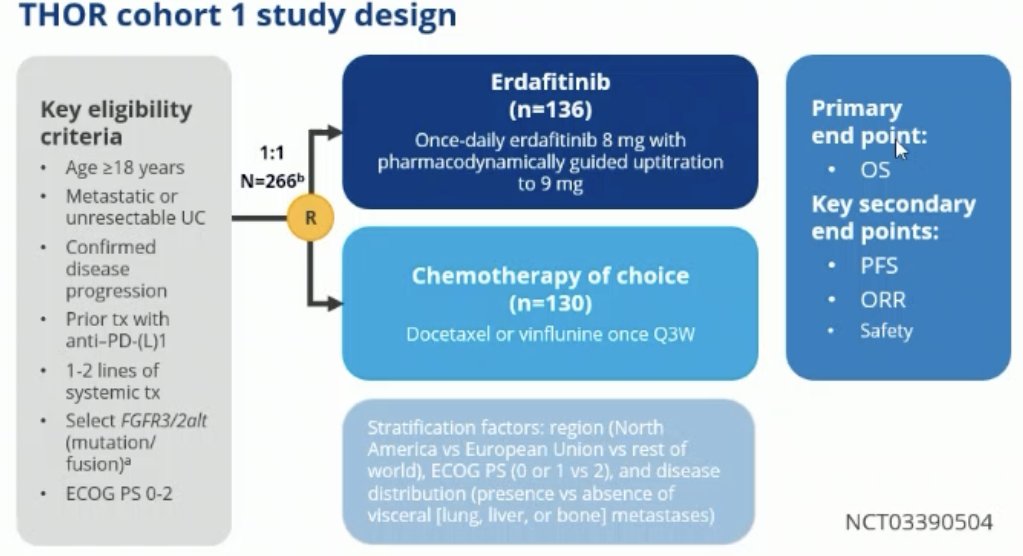
THOR Cohort 1 included adult patients with metastatic or unresectable urothelial carcinoma with confirmed disease progression following prior anti-PD-(L)1 therapy. All patients had received 1-2 lines of systemic therapy and had select FGFR3/2alt (mutation or fusion). Patients in this trial underwent 1:1 randomization to either erdafitinib (n=136) or chemotherapy of choice (n=130), typically docetaxel or vinflunine once every 3 weeks. The primary endpoint was overall survival, with key secondary endpoints of progression-free survival (PFS), objective response rate (ORR), and safety.
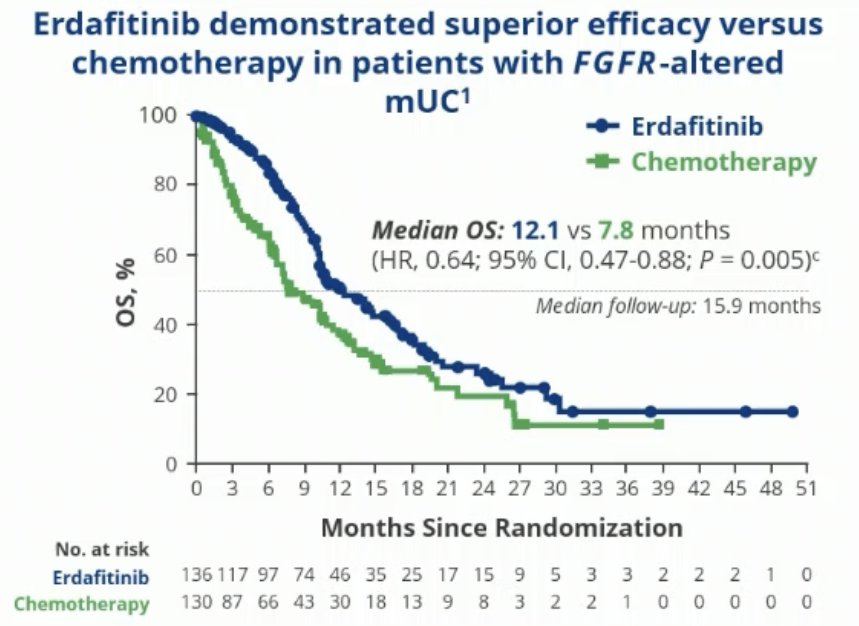
In the primary analysis, erdafitinib was shown to prolong median OS by just over 4 months (12.1 versus 7.8 months; HR: 0.64, 95% CI: 0.47 – 0.88, p=0.005). The median PFS was prolonged by 3 months (5.6 versus 2.7 months; HR: 0.58, 95% CI: 0.44 – 0.78, p=0.0002), and the ORR was 45.6% with erdafitinib versus 11.5% in the control arm (RR: 3.94, 95% CI: 2.37 – 6.57, p<0.001).
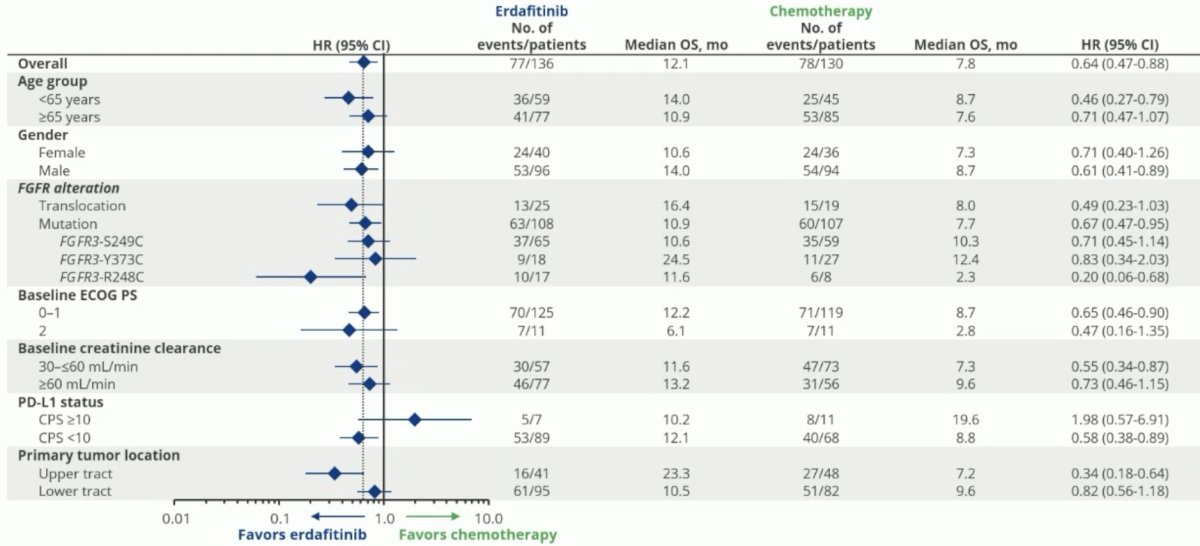
The forest plot below demonstrates that erdafitinib was associated with OS benefits across all evaluable subgroups, with Dr. Loriot highlighting that benefits were noted across all evaluable FGFR alterations. Only 7 patients were PD-L1 positive, limiting any conclusions in this subgroup.
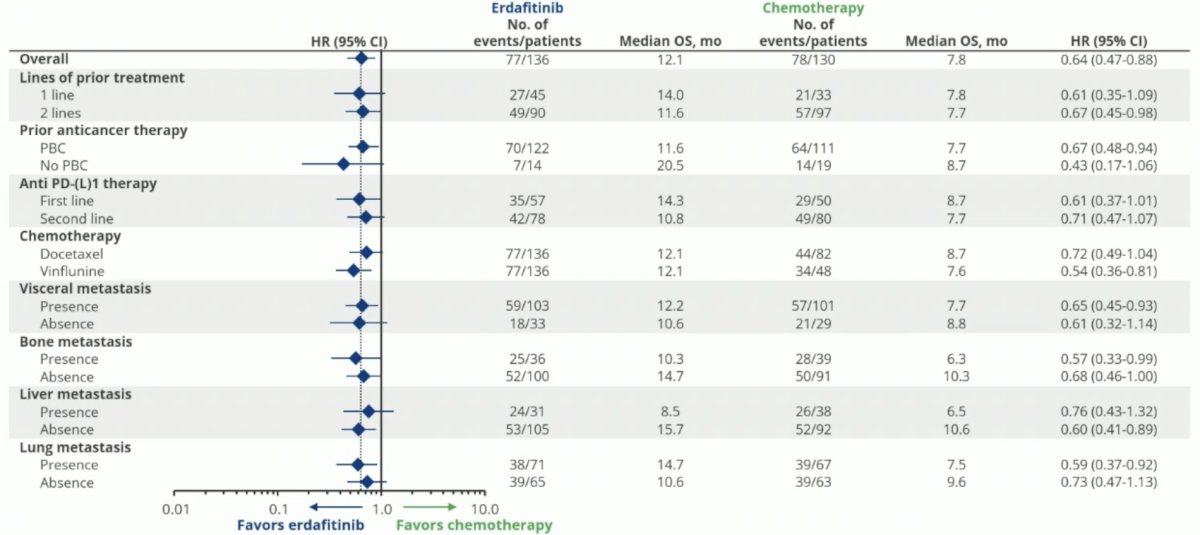
Benefits with erdafitinib were observed irrespective of the number of lines of prior treatment, whether patients received platinum-based chemotherapy or not, receipt of anti-PD-(L)1 therapy in the 1st or 2nd line settings, and site of visceral metastasis.
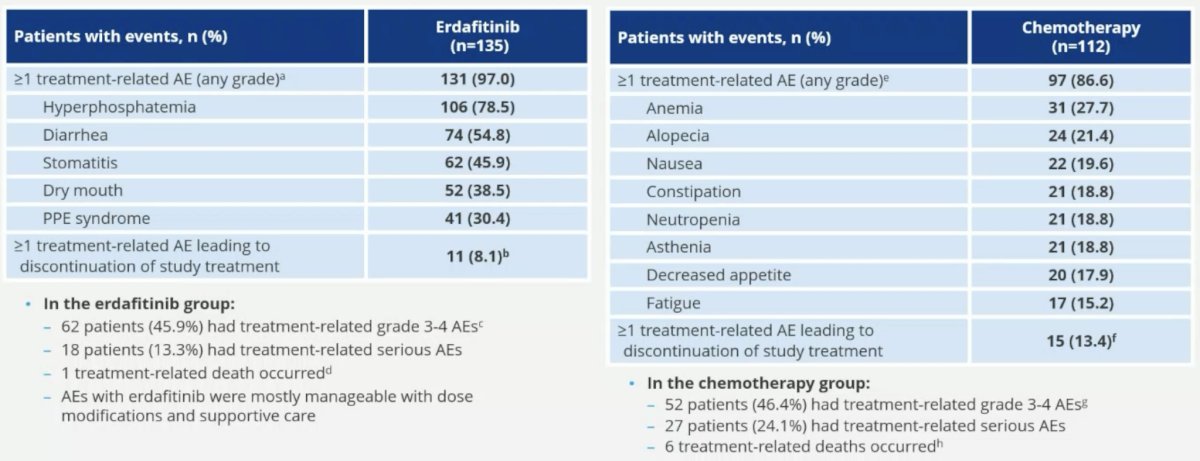
The safety profiles were consistent with the known profiles of erdafitinib and chemotherapy.
Dr. Loriot concluded as follows:
- Erdafitinib significantly extends overall survival in patients with advanced/metastatic urothelial carcinoma with FGFRalt following prior treatment with anti-PD-(L)1, with a median overall survival of 1 year (4 months improvement; HR: 0.64)
- This overall survival benefit of erdafitinib was generally consistent across clinically relevant subgroups, including age, FGFRatl type (mutations versus fusions), and primary tumor location (upper versus lower tract).
- The overall survival benefit in subgroups should be interpreted cautiously:
- This study was not designed to assess treatment effects in subgroups
- The number of patients in some subgroups was very limited (e.g., PD-L1 high, ECOG PS 2).
- The safety profile of erdafitinib was consistent with that observed in the BCL2001 study and compared favorably with that of chemotherapy
- The study results support erdafitinib as a standard of care option for patients with metastatic urothelial carcinoma with FGFRalt following anti-PD-(L)1 treatment.
Presented by: Yohann Loriot, MD, PhD, Medical Oncology Department, Gustave Roussy, Université Paris-Saclay, Paris, France.
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
- Bellmunt J, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017:376(11):1015-1026.
- Morgans AK, et al. Clinical and Patient-Reported Outcomes of Advanced Urothelial Carcinoma Following Discontinuation of PD-1/L1 Inhibitor Therapy. Clin Genitourin Cancer. 2022;20:543-552.
- Loriot Y, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med. 2019;381(4):338-348.
- Siefker-Radtke AO, et al. Efficacy and safety of erdafitinib in patients with locally advanced or metastatic urothelial carcinoma: long-term follow-up of a phase 2 study. Lancet Oncol. 2022;23(2):248-258.


