(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a urothelial cancer abstracts poster session. Dr. Nicholas Sayegh presented the results of a real-world effectiveness study of single agent enfortumab vedotin (EV) in patients with locally advanced or metastatic urothelial carcinoma based on the line of therapy and impact of prior platinum-based chemotherapy and PD-1/PD-L1 inhibitors.
EV is a Nectin-4-directed antibody and microtubule inhibitor conjugate indicated as monotherapy for the treatment of patients with metastatic urothelial carcinoma who have previously received both a PD-1/L1 inhibitor and platinum-based chemotherapy or are ineligible for cisplatin-containing chemotherapy and have received ≥ 1 prior lines of therapy. This is based on the results of EV-301 which was a global, open-label phase 3 trial that demonstrated that EV, compared to investigator-chosen chemotherapy (standard docetaxel, paclitaxel, or vinflunine), was associated with overall survival benefits in this setting (median OS: 13 versus 9 months; HR: 0.70, 95% CI: 0.56 to 0.89, p=0.001).1 However, assessment of clinical trial outcomes in the real world remains of utmost clinical significance. As such, the objective of this study was to evaluate the ‘real world’ effectiveness of EV, based on the line of therapy and impact of prior treatment.
This study used the nationwide (US-based) Flatiron Health Electronic Health Record-derived de-identified database. The study included patients with a diagnosis of advanced, recurrent, or metastatic urothelial carcinoma of the upper or lower urinary tract and who were treated with single agent EV in the 2nd line setting or beyond after December 18, 2019 (FDA accelerated approval date). Exclusion criteria included absence of documentation of 1st line therapy or absence of evidence of contact for 90 days from diagnosis of metastatic urothelial carcinoma at the treating institution (to ensure patients were actively engaged in care at the institution providing data).
Study outcomes included the time to next therapy and overall survival (OS), which were summarized based on the line of therapy and receipt of prior platinum-based chemotherapy and PD-1/L1 inhibitors, using Kaplan Meier survival estimates and associated 95% confidence intervals (CI).
This analysis included a total of 431 eligible patients, treated between January 2020 and September 2022.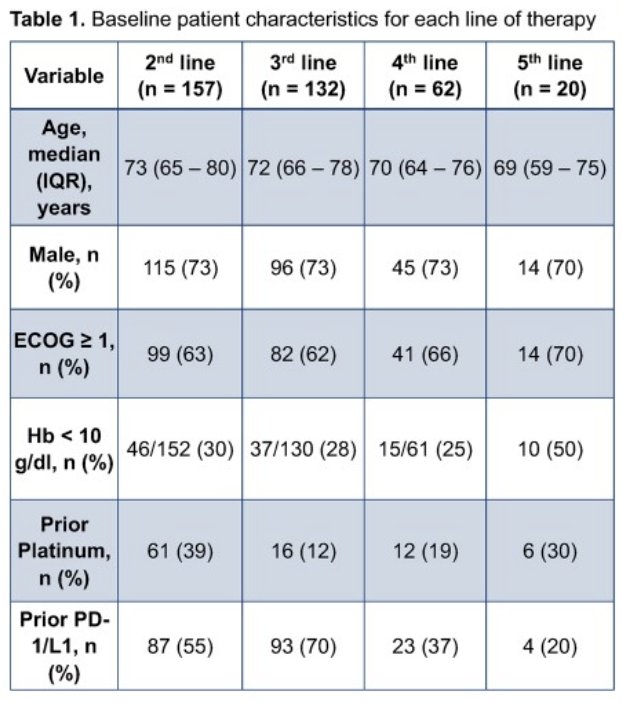
The study outcomes are summarized in the table below. While there are significant between-group differences in baseline patient characteristics, it is clear that EV maintains relative efficacy irrespective of line of therapy administered and whether prior platinum-based chemotherapy or PD-1/PD-L1 inhibitors were previously administered.
Based on these results, Dr. Sayegh concluded that EV retains activity in patients with metastatic urothelial carcinoma, regardless of prior receipt of platinum-based chemotherapy and PD-1/L1 inhibitors and line of systemic therapy. These data may further aid with patient counseling, prognostication, and selection of therapy in the clinics.
Presented by: Nicolas Sayegh, MD, Post-doctoral Research Fellow, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
Reference:- Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N Engl J Med 2021 Mar 25;384(12):1125-1135.


