(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a urothelial cancer abstracts poster session. Dr. Philippe Barthelemy presented the results of a subgroup analysis of AVENANCE, a real-world study of avelumab 1st line maintenance in advanced urothelial carcinoma.
In June 2020, the US Food and Drug Administration (FDA) approved avelumab for the maintenance treatment of patients with locally advanced or metastatic urothelial carcinoma that had not progressed with 1st line platinum-containing chemotherapy. This approval followed the initial results of the JAVELIN-100 trial that demonstrated an overall survival improvement from 14.3 months in the best supportive care arm to 21.4 months in the avelumab + best supportive care arm (HR: 0.69, 95% CI: 0.56 to 0.86, p=0.001).1 As such, avelumab maintenance has become guideline recommended in this setting.
Despite the publication of level one evidence for avelumab in patients with advanced urothelial carcinoma, there is often a discord between clinical trials and real-world outcomes. Reasons for such discrepancies include patients in clinical trials classically being healthier patients with superior performance status and easier access to 2nd line, salvage therapies. Furthermore, clinical trials often have strict eligibility criteria that limit external generalizability/validity of the study results. As such, real world data for avelumab, and other approved drugs in the advanced disease space, are of utmost importance.
It is estimated that approximately 10% of bladder cancers have a non-pure urothelial carcinoma histology. These tumors are often underdiagnosed and appropriate treatment for such patients remains an unmet clinical need. AVENANCE (NCT04822350) is evaluating avelumab 1st line maintenance in patients with advanced urothelial carcinoma in France and has previously shown clinical activity in a heterogenous population (N=593).2 In this study, the authors report on the outcomes of a subgroup analysis of patients with histological variants.
Study eligible patients included those with advanced urothelial carcinoma that had not progressed on 1st line platinum-based chemotherapy and had previously, currently receiving, or planned for avelumab 1st line maintenance. Analysis was restricted to non-pure urothelial carcinoma histology (mixed urothelial carcinoma with variants and pure variants). The primary outcome was overall survival, with secondary outcomes including:
- Progression-free survival
- Duration of treatment
- Safety
Of the 593 patients, 44 (7.4%) met the eligibility criteria. The median follow-up was 18 months (range: 12.9 to 20.4 months). 73% of patients had urothelial carcinoma with variants (including micropapillary and epidermoid) and 27% had pure variants. At platinum-based chemotherapy initiation, most patients had metastatic disease (92 – 97%) and ECOG performance status of 0 – 1 (56 – 71%). The most common chemotherapy regimen was carboplatin + gemcitabine (66 – 75%). The best response to platinum-based chemotherapy by histology were as follows:
- Urothelial carcinoma with variants:
- Complete response: 19%
- Partial response: 59.4%
- Stable disease: 22%
- Pure variants:
- Complete response: 33.3%
- Partial response: 58,3%
- Stable disease: 8.3%

The median duration of treatment was 5.1 months (95% CI: 2.8 – 17.4 months), and 34.1% were still receiving treatment at the data cut-off. The 12-months OS rate was 69%, compared to 65% in the overall, full cohort population of 593 patients. PFS was also comparable at 5.6 – 5.7 months.
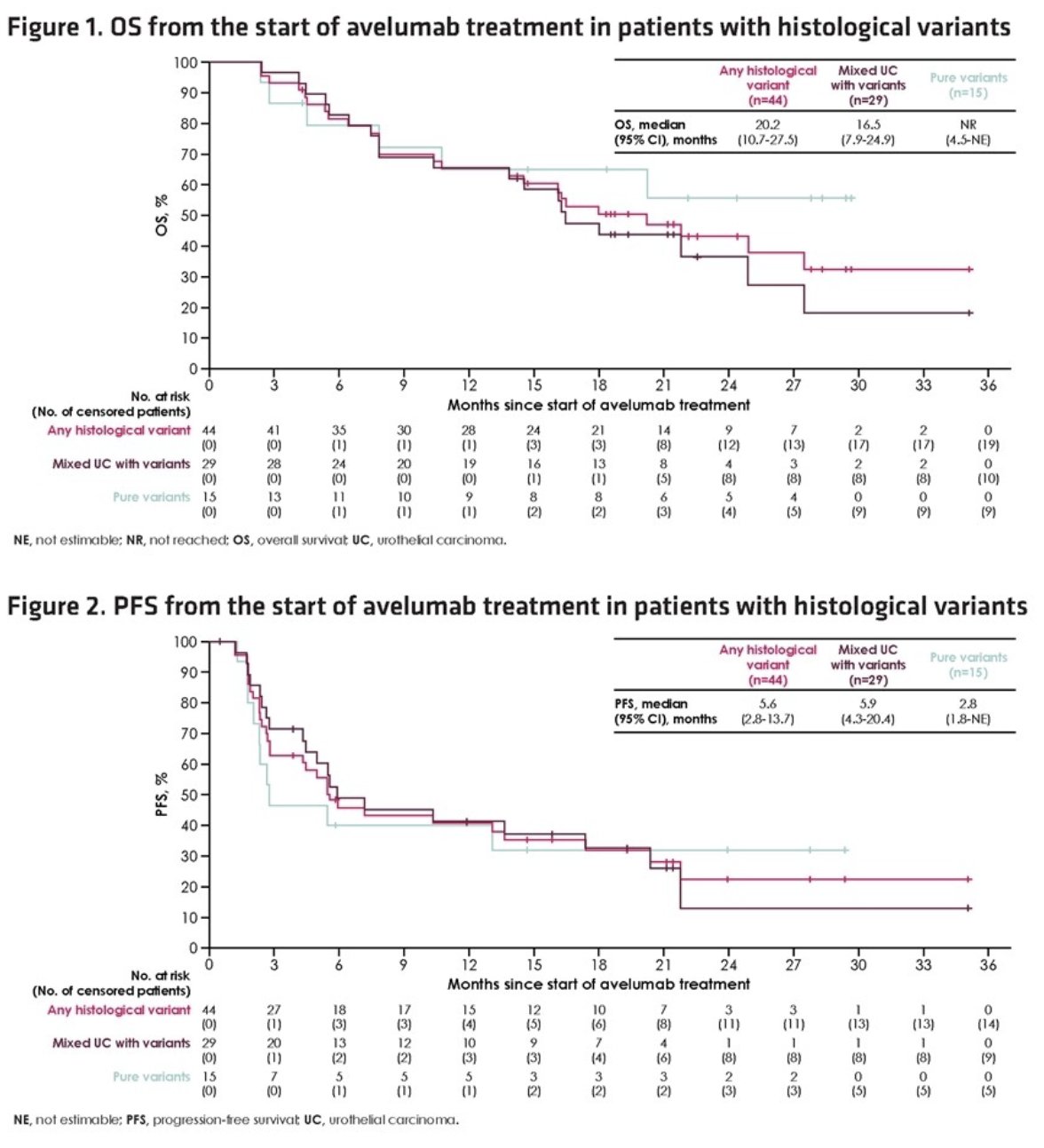
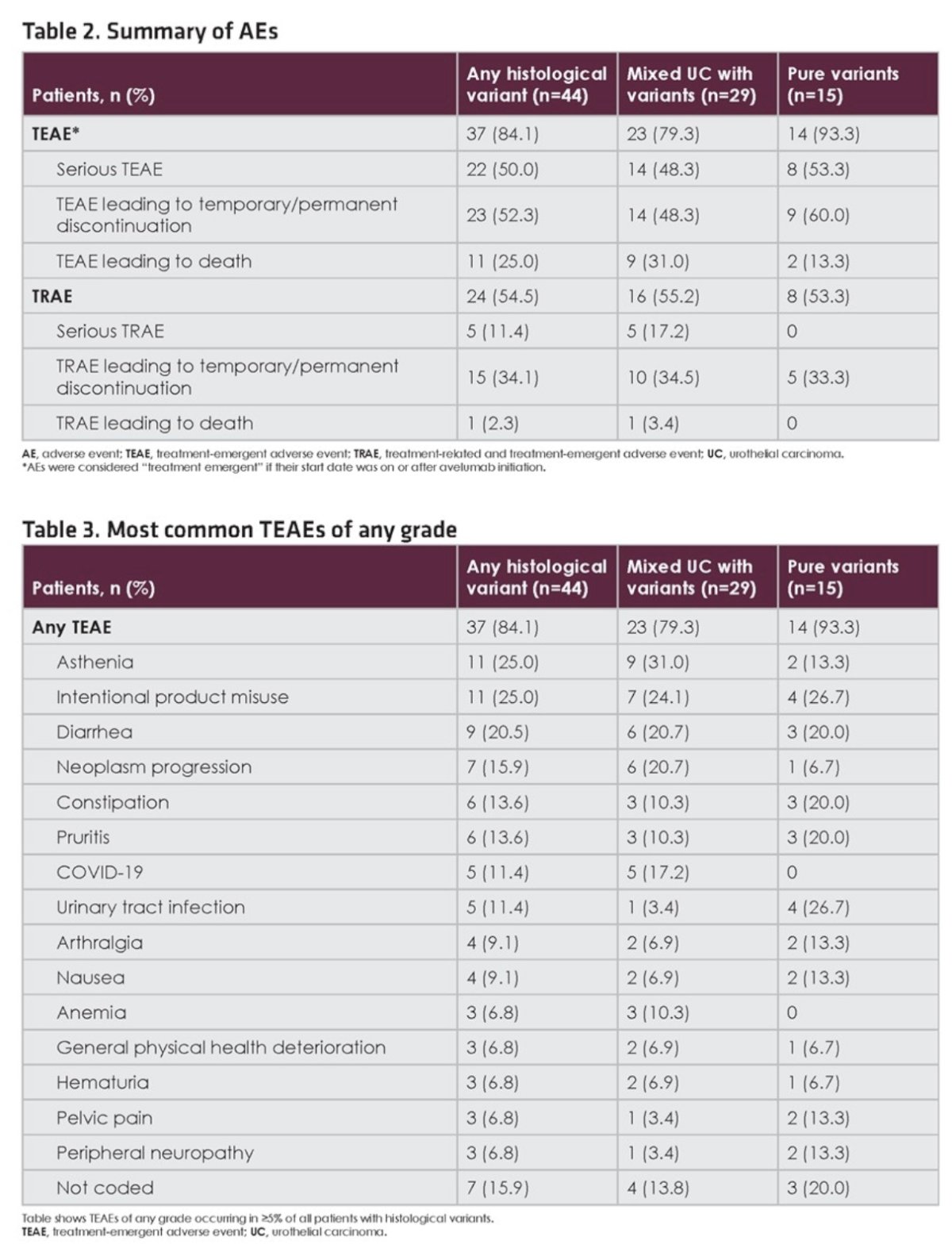
The proportion of patients with any-grade or serious treatment-related adverse events was also comparable.
Based on these results, Dr. Barthelemy concluded that:
- Real-world data from AVENANCE provide evidence for the effectiveness and safety of avelumab 1st line maintenance in patients with advanced urothelial carcinoma with histological variants that had not progressed following 1st line platinum-based chemotherapy.
- To date, this is the first analysis of avelumab 1st line maintenance in this patient population
- These subgroup data are consistent with findings in the overall AVENANCE population and further support the recommendation of avelumab 1st line maintenance as standard of care in patients with advanced urothelial carcinoma following disease control with 1st line platinum-based chemotherapy, including those with histological variants
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1103-1115.
- Barthelemy P, Loriot Y, Voog E, et al. Full analysis from AVENANCE: A real-world study of avelumab first-line (1L) maintenance treatment in patients (pts) with advanced urothelial carcinoma (aUC). J Clin Oncol 2023;41(6):471-471.


