(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a presidential session. Dr. Michiel van der Heijden presented the results of CheckMate 901, a phase III trial evaluating the combination of nivolumab plus gemcitabine/cisplatin versus gemcitabine/cisplatin alone for patients with previously untreated unresectable or metastatic urothelial carcinoma.
Cisplatin-based chemotherapy remains a 1st line standard of care option for eligible patients with unresectable or metastatic urothelial carcinoma. The response rates are in excess of 40% with median overall survival (OS) of ~15 months; however, durable responses are rare. Prior to the presentation of EV-302/KEYNOTE-A39 by Dr. Powles, no novel agent has improved OS when concurrently added to platinum-based chemotherapy in the 1st line treatment of unresectable or metastatic urothelial carcinoma, although avelumab has been approved in the ‘sequential’ maintenance setting for patients without evidence of disease progression with platinum-based chemotherapy.1 As such, there remains an unmet clinical need in the 1st line setting for treatments that improve patient outcomes. In this study, Dr. van der Heijden presented the final results of CheckMate 901, which was concurrently published today in The New England Journal of Medicine,2 evaluating gemcitabine-cisplatin +/- nivolumab in cisplatin-eligible patients with previously untreated unresectable or metastatic urothelial carcinoma.
608 patients in this trial underwent 1:1 randomization, stratified by tumor PD-L1 expression and presence/absence of liver metastases, to either:
- Nivolumab 360 mg on D1 + gemcitabine (1,000 mg/m2) on D1/8 + cisplatin (70 mg/m2) on D1 in 3-week cycles, up to a total of 6 cycles
- Nivolumab maintenance at 480 mg every 4 weeks was continued as maintenance therapy in responders until progression, unacceptable toxicity, withdrawal, or up to 24 months
- Gemcitabine + cisplatin at the same doses/schedule/cycles
The co-primary endpoints were OS and PFS, per BICR, The median study follow-up was 33.6 months, ranging between 7.6 and 62.4 months.
Statistical considerations and analyses are summarized in the slide below:
Baseline patient characteristics were well-balanced between the two study arms. The median patient age was 65 years. The primary tumor site was the bladder in 75% of patients. 37% of patients had a high tumor PD-L1 expression, defined as ≥1%. 21% of patients had evidence of liver metastases.
The median duration of study therapy was 7.4 months in the nivolumab + gem/cis arm, compared to 3.7 months in the gem/cis arm. 74% of patients in the nivo + gem/cis arm completed all 6 cycles of therapy, compared to 55% of those in the control gem/cis arm. In total, 244 patients (80%) of patients randomized to nivo + gem/cis went on to receive maintenance nivolumab monotherapy. At time of data cut-off, 8% completed monotherapy and 9% were still on therapy.
The study met its primary endpoint of OS, with a median improvement of nearly 3 months (21.7 versus 18.9 months; HR: 0.78, 95% CI: 0.63 – 0.96, p=0.017).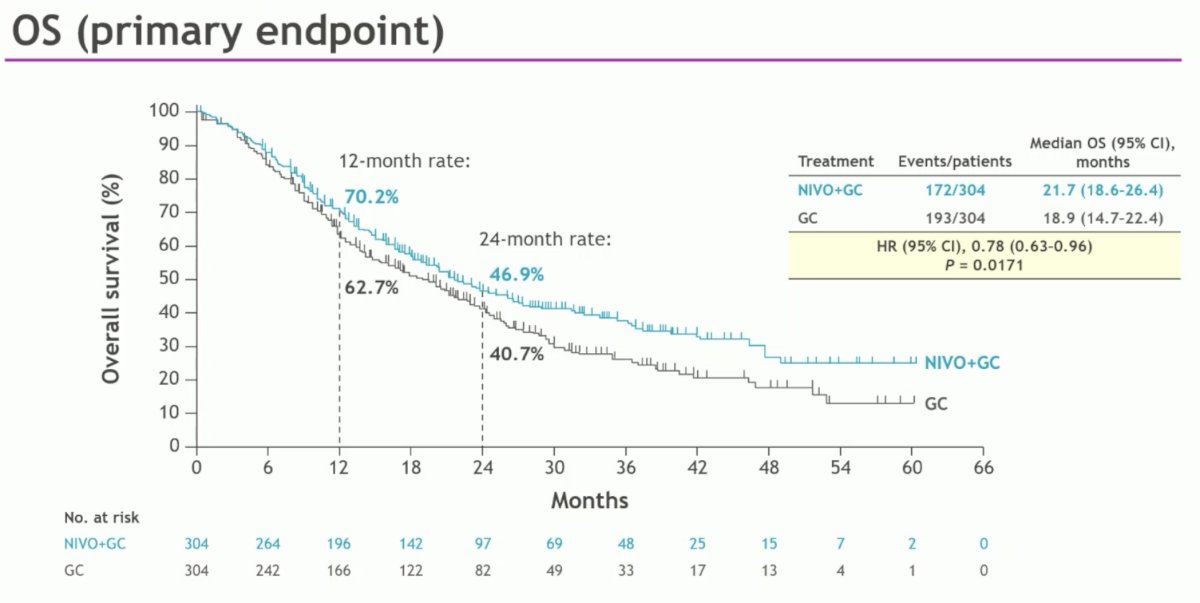
OS subgroup analyses favored almost all evaluable subgroups; however, we do note that there appeared to be an OS benefit in favor of gem-cis control in the US-treated patients (n=40; HR: 1.92, 95% CI: 0.95 – 3.88).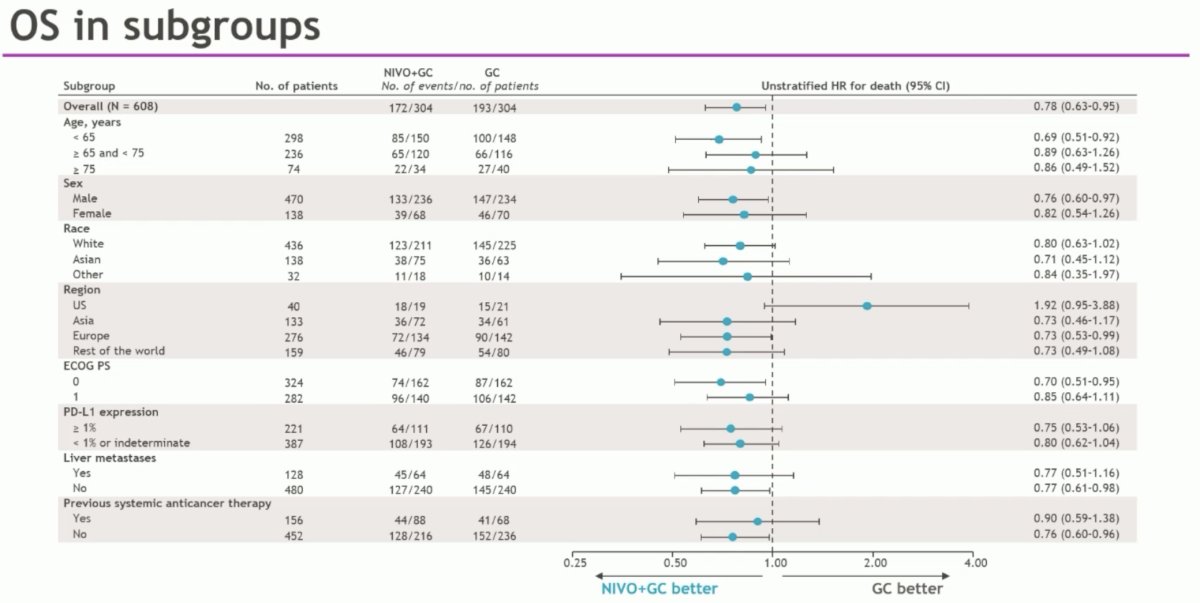
The other co-primary endpoint of PFS per BICR was also significant with improvement in median PFS from 7.6 to 7.9 months (HR: 0.72, 95% CI: 0.59 – 0.88, p=0.0012).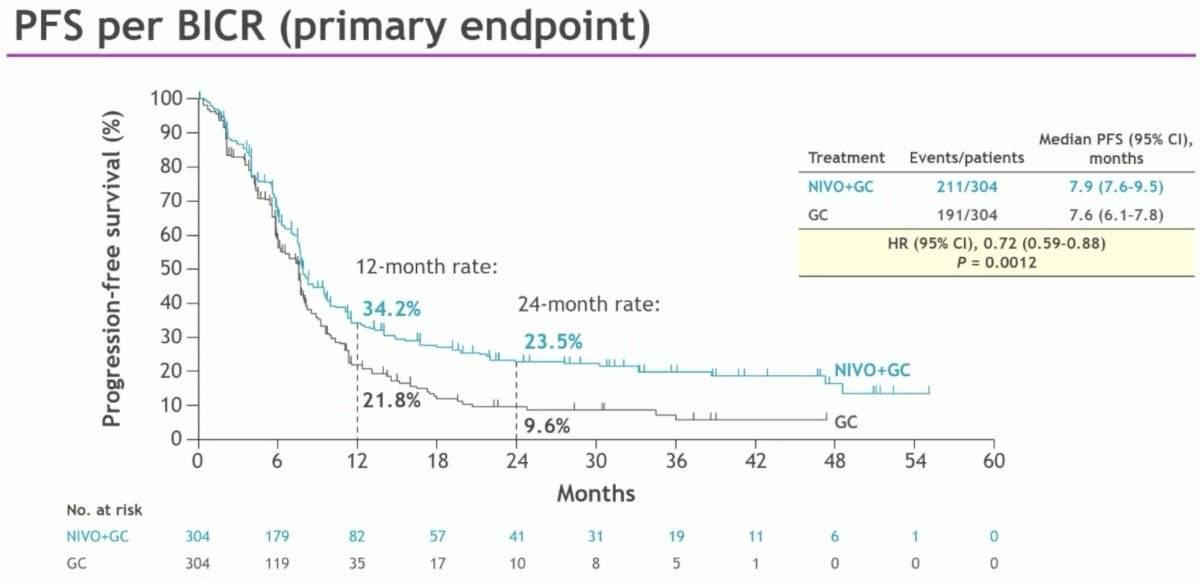

For the primary analysis of PFS, patients receiving subsequent anti-cancer therapy prior to disease progression (nivo + gem/cis: 8%; gem/cis: 24%) were censored. Immunotherapies were the most commonly received subsequent therapies prior to disease progression in the gem/cis arm. PFS sensitivity analysis not censoring those patients receiving subsequent anti-cancer therapy before disease progression demonstrated consistent PFS benefits in favor of nivo + gem/cis.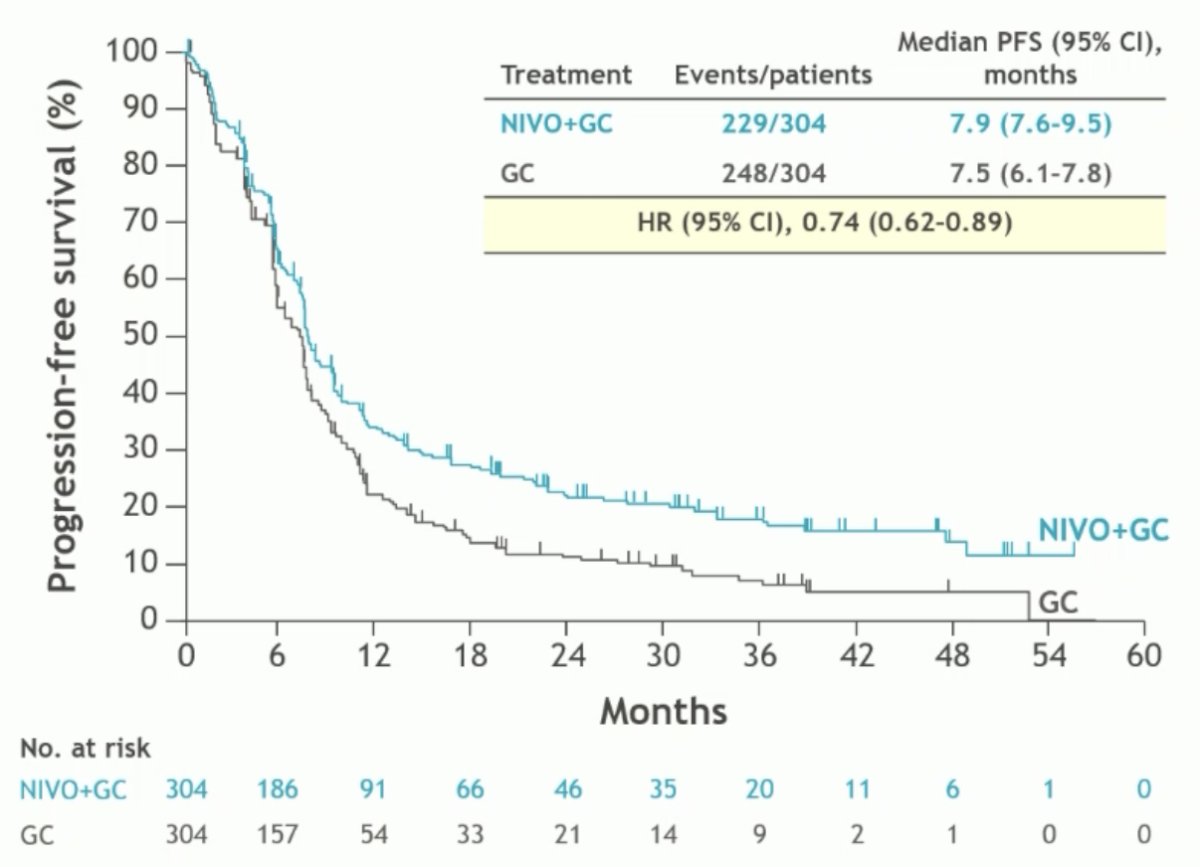
The objective response rate (ORR) was higher in the experimental arm (58% versus 43%), with a higher proportion of patients achieving a complete response with nivo + gem/cis (22% versus 12%
The median time to response was 2.1 months in both arms, with a median duration of response of 9.5 and 7.3 months in the experimental and control arms, respectively.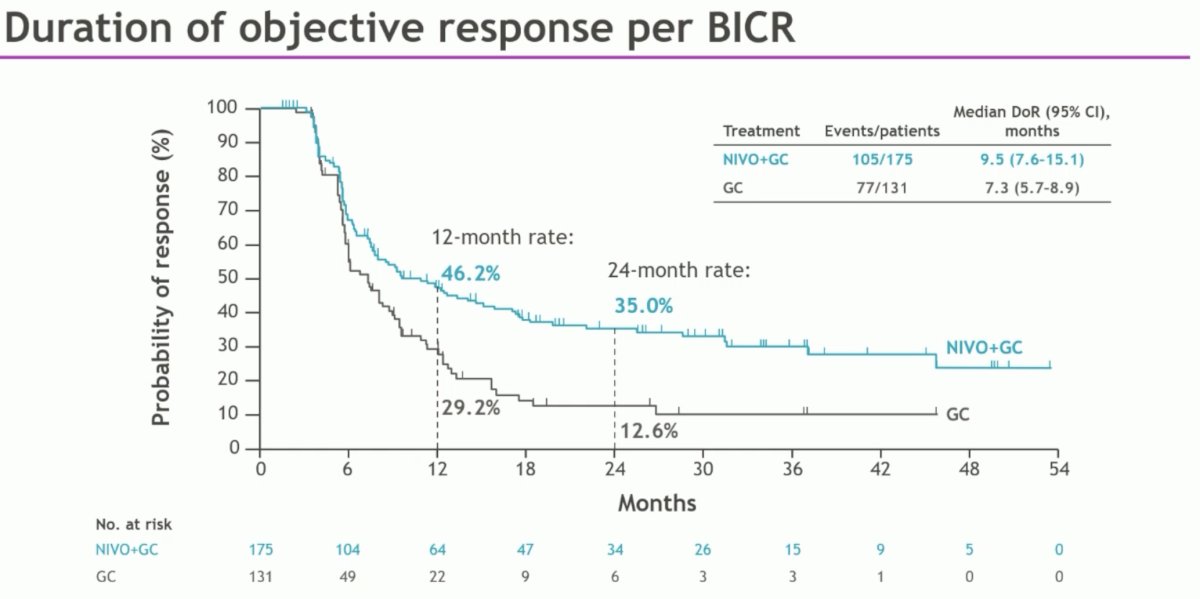
The proportions of patients receiving subsequent surgery, radiotherapy, and/or platinum-based chemotherapy were similar in the two arms. 8% of patients in the nivo + gem/cis arm received immune checkpoint inhibitors subsequent to study therapy, compared to 40% of those in the gem/cis arm.
Grade 3+ treatment-related adverse events (TRAEs) occurred in 62% and 52% of patients in the experimental and control arms, respectively. Those leading to treatment discontinuation occurred in 11% and 8%, respectively. The most common TRAEs with nivo + gem/cis were anemia (57%), nausea (47%), and neutropenia (31%).
Health related quality of life (EORTC QLQ-C30) was stable and equivalent in both arm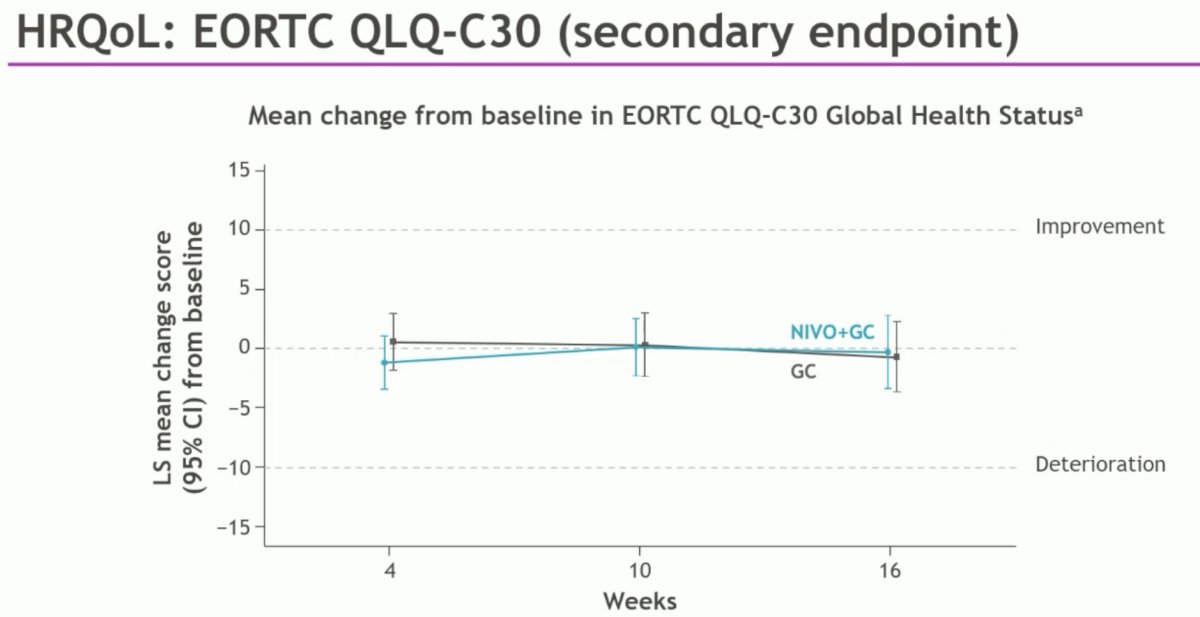
Dr. van der Heijden concluded that:
- Nivolumab + gemcitabine/cisplatin demonstrated statistically significant and clinically meaningful improvements in overall and progression-free survivals versus gemcitabine/cisplatin alone as 1st line treatment for unresectable or metastatic urothelial carcinoma
- Objective response and complete response rates were notably higher with nivolumab + gemcitabine/cisplatin, and this combination was associated with deep and durable responses
- The complete response rate was nearly doubled (22% versus 12%) and the duration of complete response was almost 3 times longer (37.1 versus 13.2 months) with nivolumab + gemcitabine/cisplatin, despite a maximum of 2 years of nivolumab treatment
- The combination of nivolumab + gemcitabine/cisplatin resulted in no new toxicity signals, and the safety profile was consistent with the established safety of these agents in prior urothelial carcinoma trials.
- Health-related quality of life was maintained with the addition of nivolumab to gemcitabine/cisplatin.
- Nivolumab + gemcitabine/cisplatin is the 1st front line concurrent immune checkpoint inhibitor plus chemotherapy combination to improve overall survival in this setting, with results supporting nivolumab plus cisplatin-based chemotherapy as a new standard of care for patients with unresectable or metastatic urothelial carcinoma
Presented by: Michiel S. van der Heijden, MD, PhD, Division of Medical Oncology, Netherlands Cancer Institute, Amsterdam, Netherlands
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Powles T, Park SH, Voog E, et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2020 Sept 24;383(13):1218-1230.
- Van der Heijden MS, Sonpavde G, Powles T, et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N Engl J Med 2023.


