(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Bernadett E. Szabados presented the results of ABACUS-2, a phase II trial evaluating the safety and efficacy of neoadjuvant atezolizumab in patients with non-urothelial, muscle invasive bladder cancer.
Pure urothelial carcinoma remains the most common subtype (~80% of total urothelial malignancies), with other subtypes including squamous, glandular, and neuroendocrine differentiation, among others.
Variant histology urothelial carcinoma subtypes demonstrate a high risk for stage progression and development of metastatic disease. Currently, there is limited evidence for the optimal treatment of invasive, ‘non-conventional’ urothelial carcinoma. The objective of the ABACUS-2 trial was to evaluate 2 cycles of neoadjuvant atezolizumab in patients with non-conventional urothelial carcinoma.
The phase II ABACUS-2 trial included patients with cT1-4aN0-1M0 mixed or pure non-conventional urothelial carcinoma subtype with residual disease following TURBT who were fit and planned for radical cystectomy. Patients must have been assessed as cisplatin-ineligible or not appropriate. Patients were planned for 2 cycles of neoadjuvant atezolizumab (1,200 mg IV every 3 weeks) followed by radical cystectomy and then standard of care follow-up for 2 years. The primary endpoint was pathologic complete response rate (pCR), with secondary endpoints of disease-free survival (DFS), overall survival (OS), safety, and planned biomarker analysis.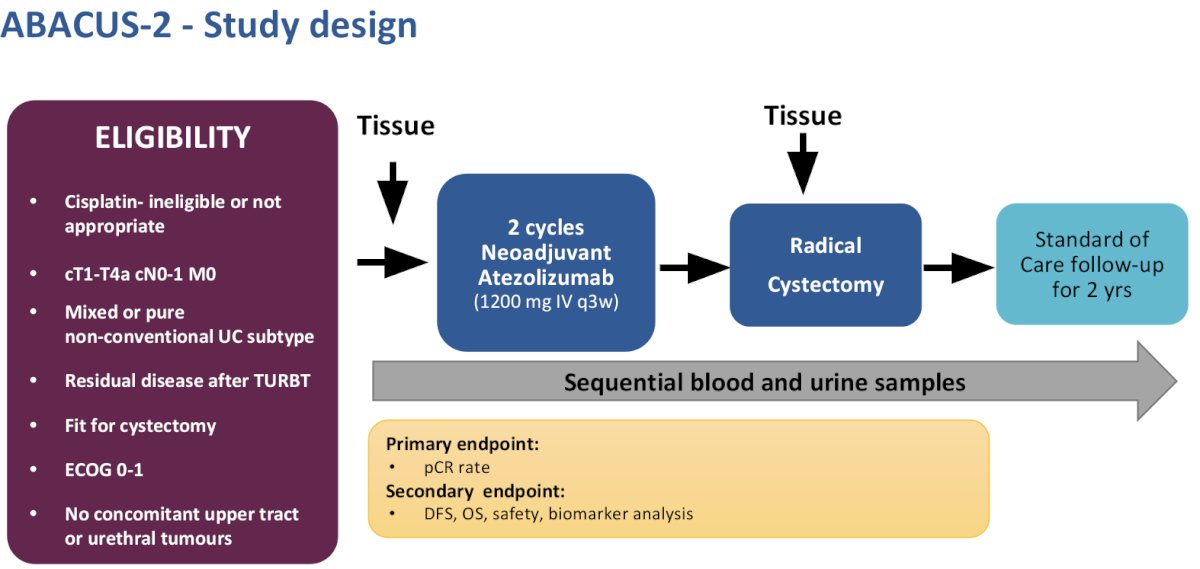
Thirty-five patients were included with a median follow-up of 10.2 months. The median patient age was 69 years. 9% had received prior BCG, and the majority had cT2 disease (66%).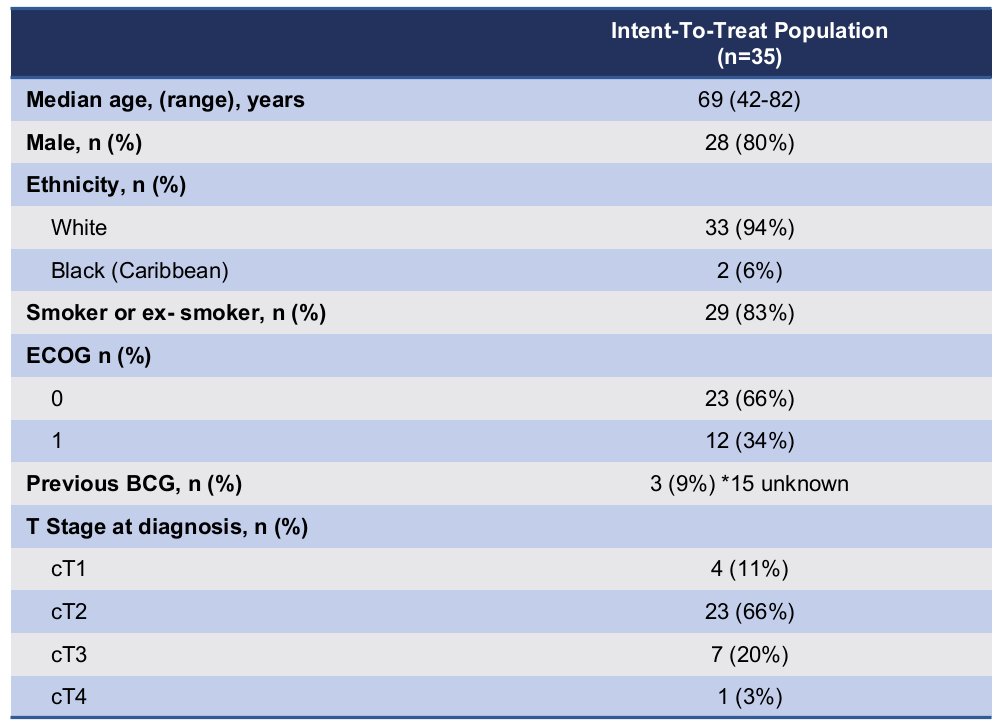
Out of the 35 recruited patients, 32 received both cycles, with the 3 remaining receiving 1 cycle only. 33/35 underwent a cystectomy, and 35 patients were included in the intent-to-treat analysis. Of these 35 patients, 24 had evaluable central pathology data for review.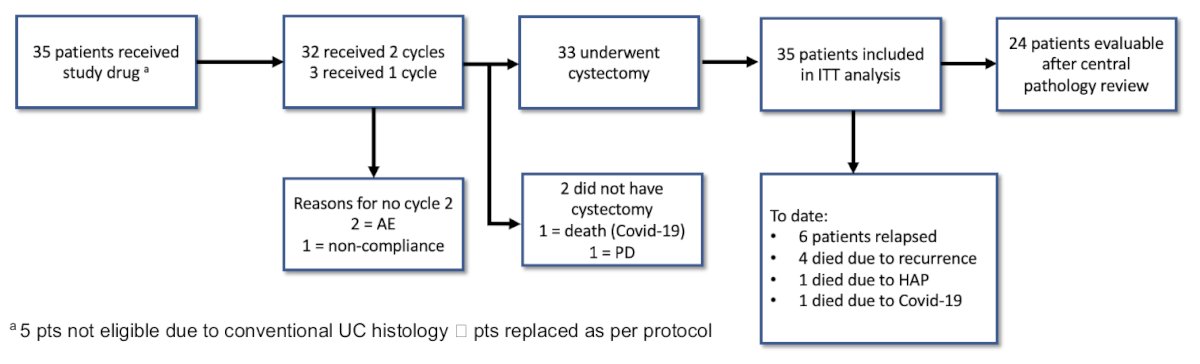
Following central pathology review, there was a significant change in the assigned variant histologies, which highlights the importance of expert review in these unique cases. The most common baseline histologic subtype remained conventional urothelial carcinoma (12/35), followed by squamous, sarcomatoid, and adenocarcinoma differentiation (5/35 for each).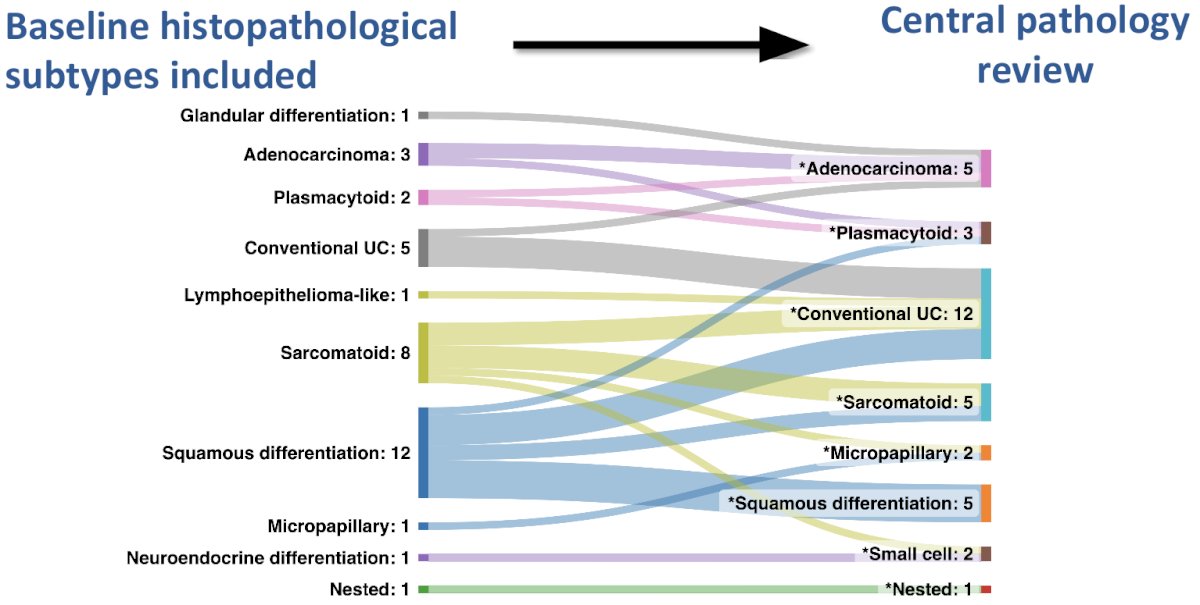
A pCR was observed as follows:
- Overall intent-to-treat population: 14/35 (40%)
- Evaluable cohort after central pathology review: 9/24 (38%)
- Glandular/adenocarcinoma: 1/6 (16.7%)
- Squamous: 1/12 (8.3%)
- Sarcomatoid: 5/8 (63%)

Grade 3-4 treatment-related adverse events occurred in only 1 patient (acute kidney injury), which prevented the patient from receiving the 2nd cycle of atezolizumab. There were no treatment-related deaths.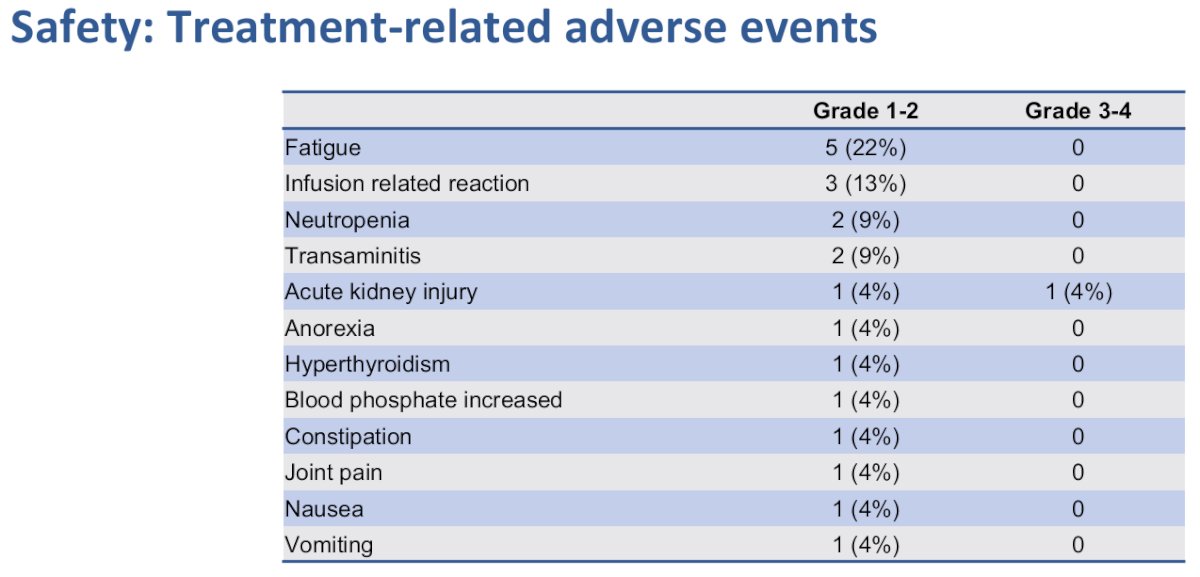
Dr. Szabados concluded as follows:
- Non-conventional urothelial carcinoma incorporates a spectrum of cancer types with different biologies. The investigators demonstrated inconsistency in their pathological assessment, complicating the analysis of a prospective study.
- Two cycles of neoadjuvant atezolizumab showed activity in non-conventional urothelial cancer subtypes with the highest efficacy in sarcomatoid urothelial cancer. This warrants further investigation. Response rates in other subtypes are less promising, although uncertainty remains due to small numbers and inconsistency in the pathology reviews.
- Treatment was safe and adverse events were in line with previous experience of neoadjuvant atezolizumab.
- Due to the results of the central pathology reviews, the trial will reopen and continue to enroll patients to test the primary endpoint more robustly.
Presented by: Bernadett E. Szabados, MD, Consultant Urological Surgeon at University College Hospital, Senior Clinical Researcher at Barts Cancer Institute, London, UK
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023


