(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Srikala Sridhar presented the results of EV-103 Cohort L, which evaluated the perioperative treatment with enfortumab vedotin (EV) monotherapy in cisplatin-ineligible patients with muscle invasive bladder cancer (MIBC).
Up to 25% of all patients diagnosed with urothelial cancers present with MIBC. The current guideline-recommended standard of care for patients with MIBC is neoadjuvant cisplatin-based chemotherapy followed by surgery, with adjuvant therapy recommended in high-risk patients.1 However, up to 50% of patients may be cisplatin-ineligible and are recommended to proceed with surgery alone. Due to the high rates of recurrence in cisplatin-ineligible patients treated with surgery alone, there remains a significant clinical unmet need for novel therapies in this setting. Previously, neoadjuvant EV in cisplatin-ineligible MIBC showed encouraging anti-tumor activity, with a pathologic complete response (pCR) proportion of 36% and pathologic downstaging of 50%.2 Importantly, there were no new safety signals, no delays to surgery, and a low incidence of grade 3 or worse EV-related treatment-emergent adverse events. EV-103 Cohort L (NCT03288545) examines a perioperative approach consisting of both neoadjuvant and adjuvant EV in cisplatin-ineligible patients with MIBC.
EV-103 Cohort L included patients with cisplatin-ineligible cT2-4aN0M0 or cT1-4aN1M0 urothelial carcinoma patients medically fit for a radical cystectomy + pelvic nodal dissection. Following enrollment, patients were given EV monotherapy (1.25 mg/kg) in 3-week cycles on days 1 and 8 for 3 cycles total. Following surgery, patients received the same regimen for 6 cycles. The primary endpoint was pCR, with secondary objectives of pathologic downstaging (pDS), event-free survival (EFS), disease-free survival (DFS), overall survival (OS), and safety and tolerability.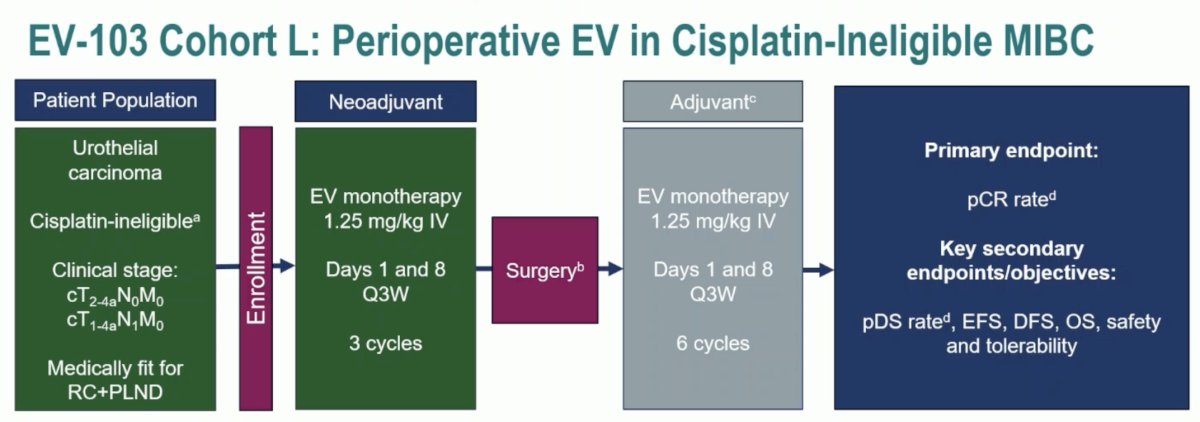
Cohort L included 51 patients. The median age was 74 years. 96% had ECOG performance status 0 – 1. The baseline stage was cT2N0 for 57% and cN+ for 10% of patients. 45% of patients had a creatinine clearance of 30 – 60 mL/min. 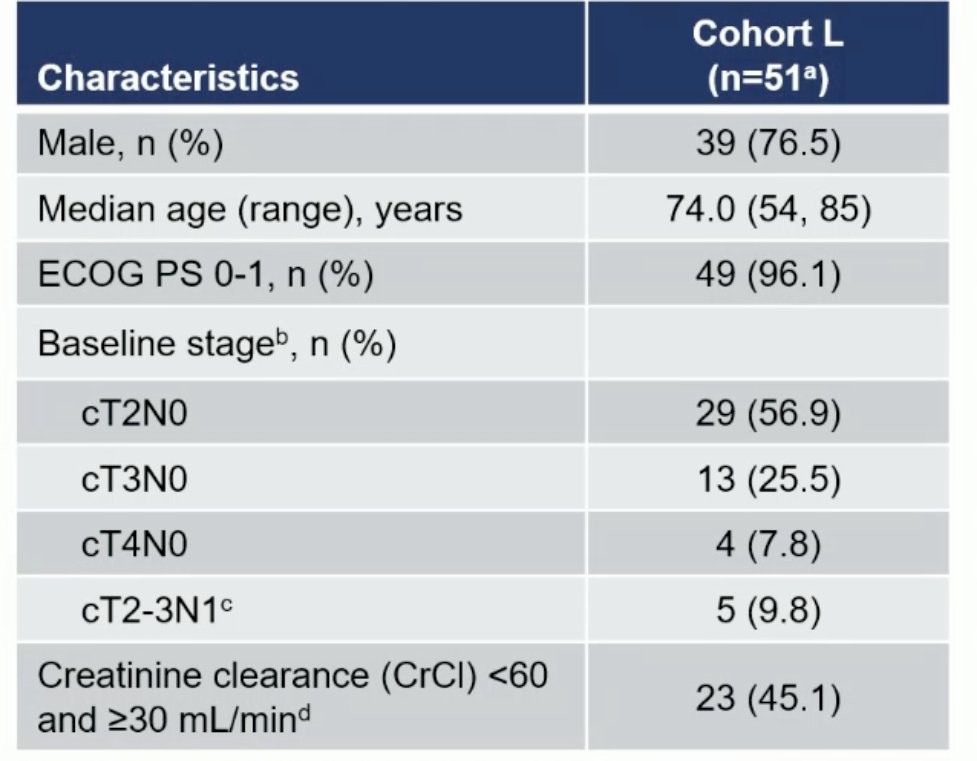
82.4% of patients completed all 3 cycles of neoadjuvant EV, with all these patients completing surgery within a median time of 1.3 months from the last EV dose.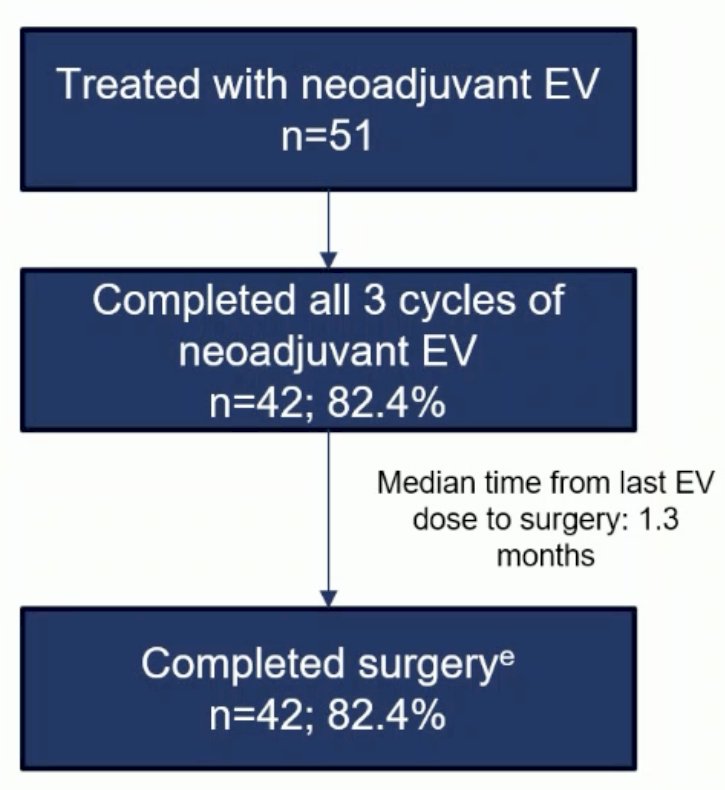
For pathologic outcomes, the pCR was 34% and pDS occurred in 42% of patients.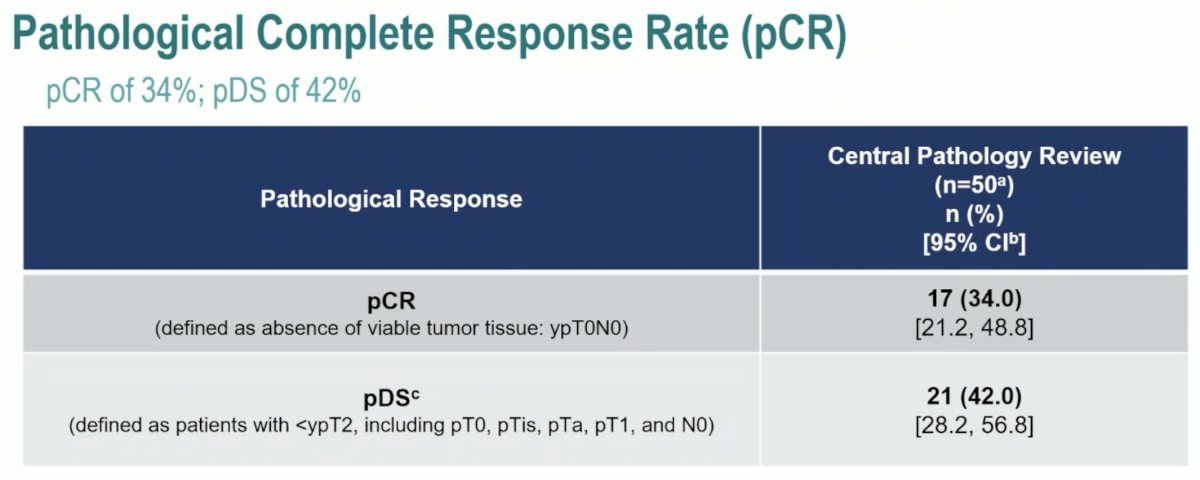
The majority of EV-related adverse events were grade 2 or lower. 57% of patients had skin reactions, with 1 patient dying of Stevens-Johnson syndrome. A third of patients had peripheral neuropathy. There were no delays to surgery secondary to EV-related treatment-emergent adverse events. The most common grade 3 or worse surgery-related adverse events were anemia, ileus, and urinary tract infection.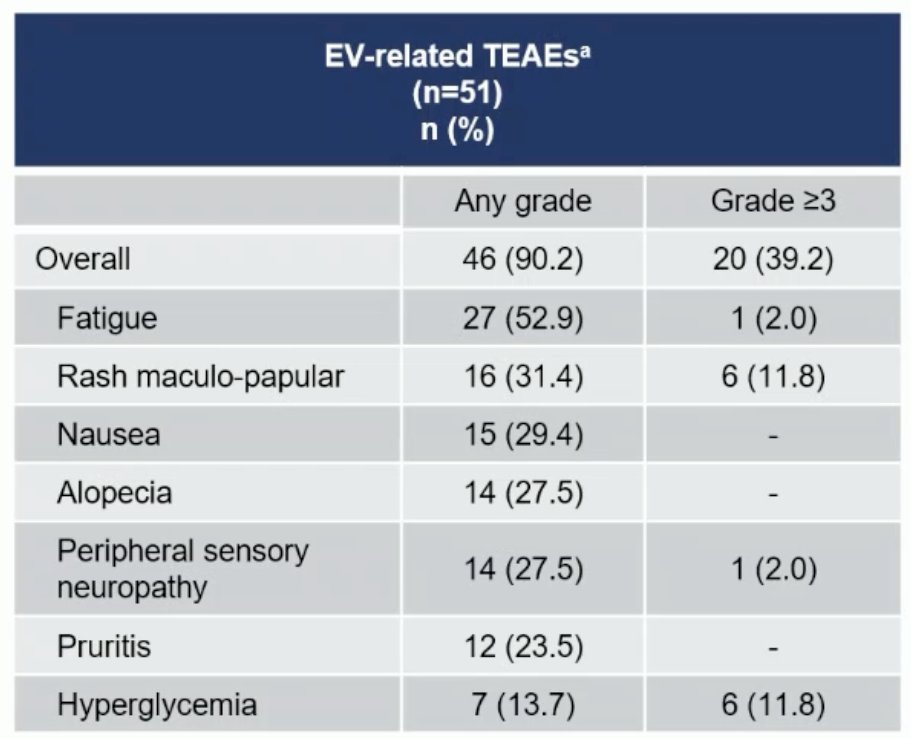
Dr. Sridhar concluded as follows:
- Enfortumab Vedotin continues to show promising antitumor activity in cisplatin-ineligible patients with muscle-invasive bladder cancer
- Pathologic complete response of 34%; pathologic downstaging rate of 42%
- The results are consistent with EV-103 Cohort H
- The safety profile of Enfortumab Vedotin was similar to previously reported data from Enfortumab Vedotin in muscle-invasive bladder cancer and locally advanced/metastatic urothelial carcinoma
- No new safety signals; but close monitoring for toxicities remains important
- No surgeries were delayed due to Enfortumab Vedotin-related treatment-emergent adverse events
- This first data disclosure from EV-103 Cohort L supports the ongoing phase 3 programs evaluating Enfortumab Vedotin in combination with pembrolizumab in MIBC (KN-905, KN-B15)
- Additional follow-up will allow characterization of event-free survival, disease-free survival, and overall survival data from Cohort L
Presented by: Srikala Sridhar, MD, MSc, FRCPC, Professor, Department of Medicine, Princess Margaret Cancer Centre, Toronto, ON
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Petrylak D, Flaig TW, Mar N, et al. Study EV-103 Cohort H: Antitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients (pts) with muscle invasive bladder cancer (MIBC) who are cisplatin-ineligible. J Clin Oncol. 2022;40:Supplement 6.


