(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate, genitourinary tumors mini oral session. Dr. Andrea Necchi presented the late-breaking abstract results from SunRISe-1 in patients with BCG-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC) receiving TAR-200 monotherapy.
The standard of care treatment for BCG-unresponsive HR NMIBC remains radical cystectomy. However, radical cystectomy is associated with significant rates of morbidity, mortality, and impact on quality of life. Additionally, many patients are either unfit for or unwilling to undergo radical cystectomy. Currently, limited treatment options are available to treat BCG-unresponsive HR NMIBC CIS, with reported 12 months complete response rates as follows:
- 19% with pembrolizumab1
- 15% with atezolizumab2
- 23% with nadofaragene firadenovec3
TAR-200 is a novel drug delivery system for the sustained local release of gemcitabine in the bladder. It is designed to address the unmet need in patients with HR NMIBC recurrence or progression following BCG treatment.
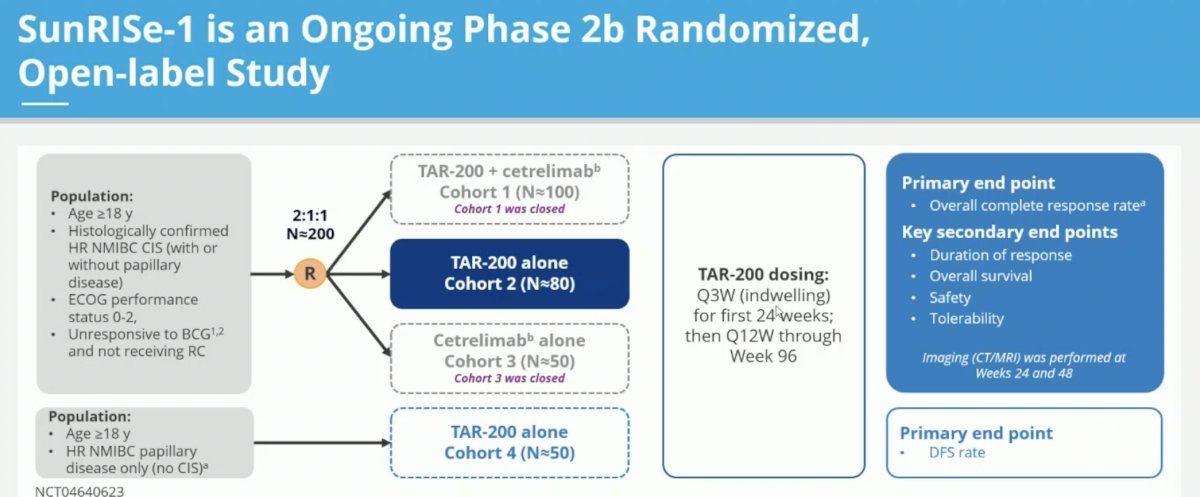
SunRISe-1 is an ongoing phase 2b randomized, open label study including adult patients with histologically confirmed HR NMIBC CIS (+/- papillary disease) unresponsive to BCG and not planned for radical cystectomy. Patients in this trial were randomized 2:1:1 to either:
- Cohort 1: TAR-200 + cetrelimab (n=100)
- Cohort 2: TAR-200 alone (n=80)
- Cohort 3: Cetrelimab alone (n=50)
Given the initial promising results with TAR-200 alone presented at AUA 2023, further enrolment in cohorts 1 and 3 was suspended. TAR-200 was dosed every 3 weeks for the first 24 weeks, followed by every 12 weeks through week 96. Complete response was determined via cystoscopy, central cytology, and central pathology at weeks 24 and 48. The primary endpoint was overall complete response rate. In this report, Dr. Necchi presented the updated results from the TAR-200 monotherapy cohort (Cohort 2) of SunRISe-1.
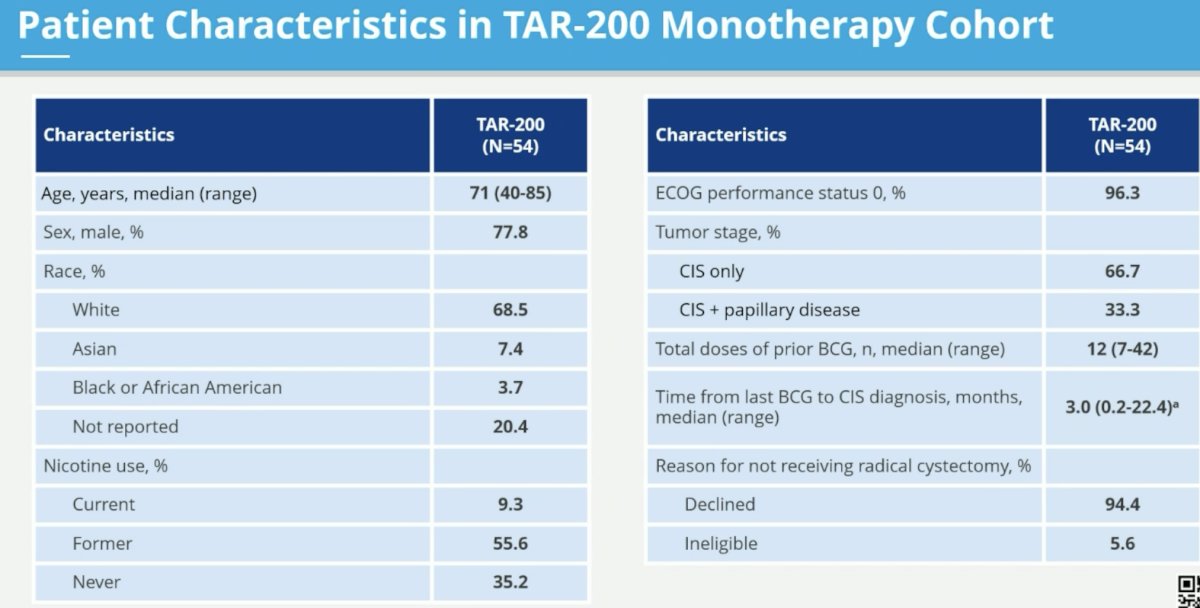
This cohort includes 54 patients to date. The median age is 71 years. CIS only disease is present in two-thirds of the cohort (remaining CIS + papillary). The median total number of doses of prior BCG is 12 (range: 7 – 42). The median time from last BCG to CIS diagnosis was 3 months.
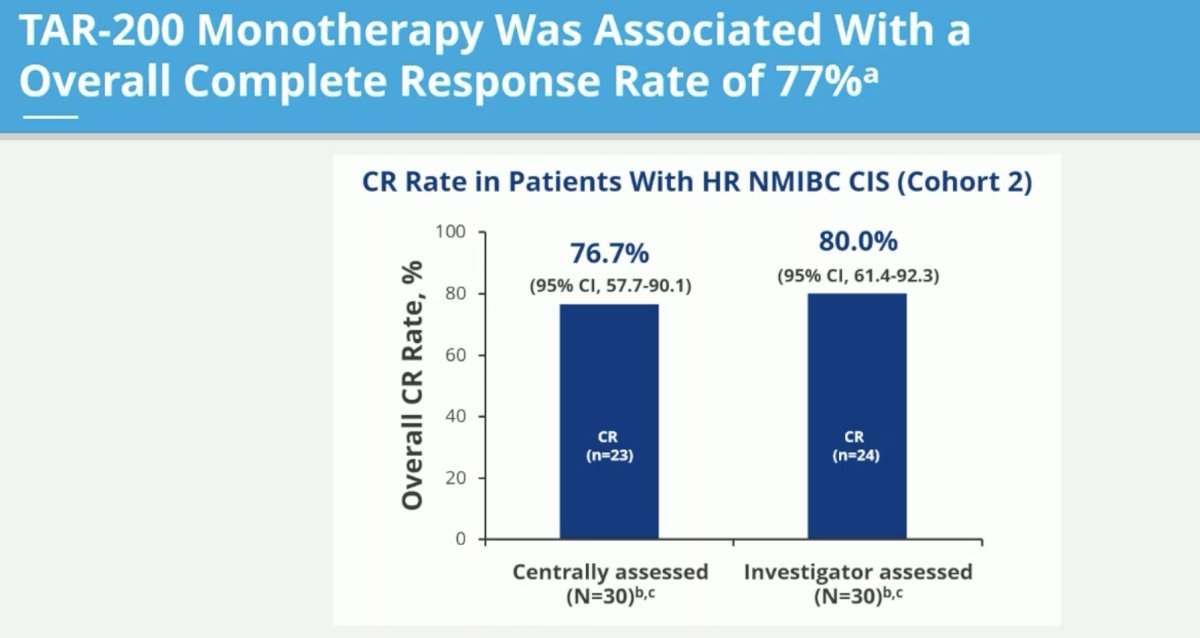
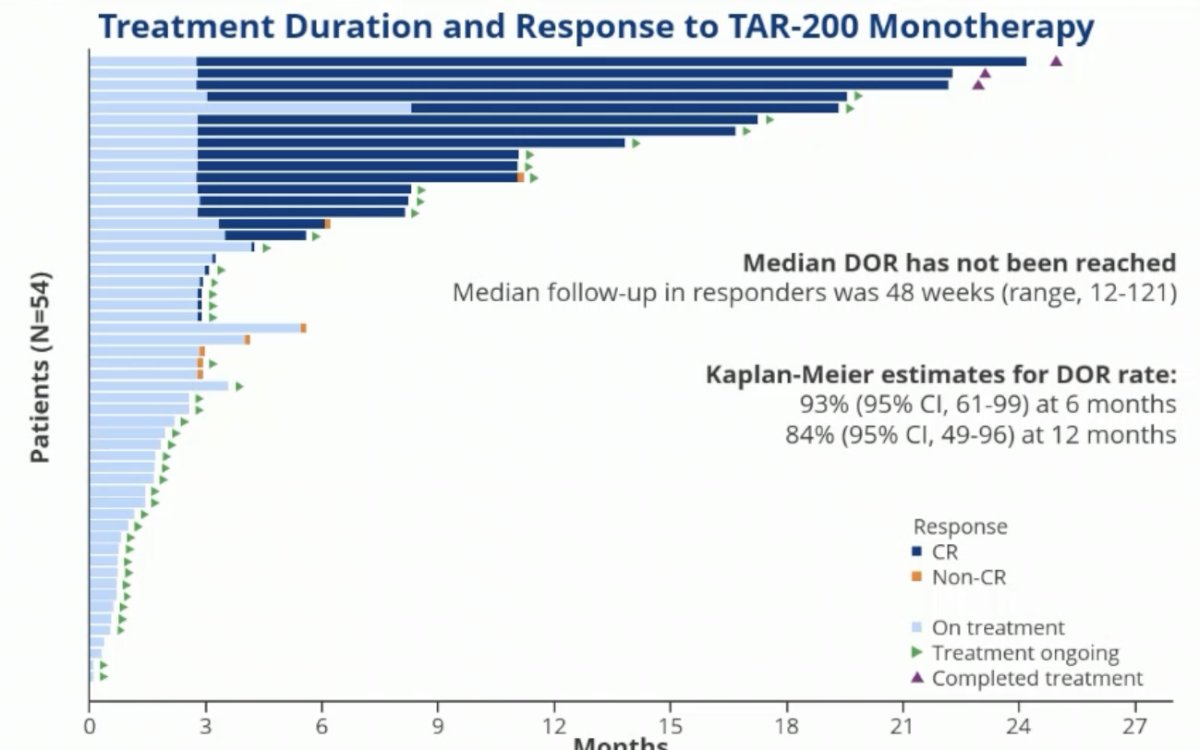
The centrally-assessed overall complete response rate was 77% (95% CI: 58 – 90%).
Of the 23 responses, 21 (91.3%) are currently ongoing. 11 patients had 6+ months duration of response, with 10 of these 11 still ongoing. A duration of response exceeding 12 months has been observed in 6 patients, all of which are still ongoing. None of the patients with a complete response have undergone a radical cystectomy.
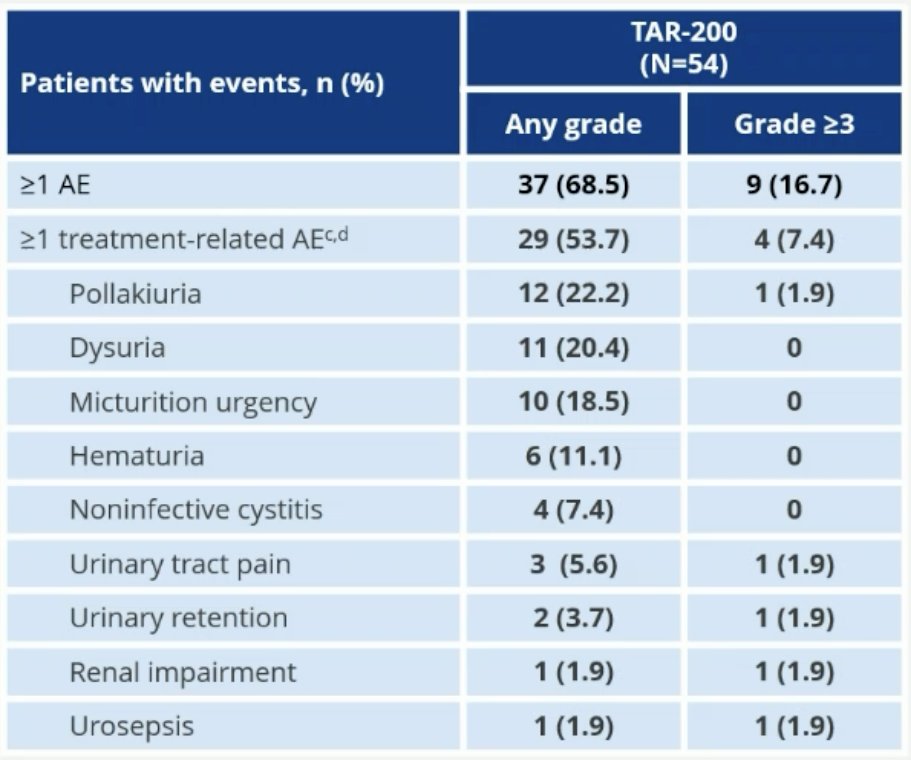
Overall, most adverse events in the TAR-200 were Grade 1 – 2. 54% of patients had 1+ treatment-related adverse events (TRAEs). 1 patient (2%) had ≥ serious TRAE. 4 patients (7.4%) had grade ≥3 TRAEs. There was a low rate of treatment discontinuation due to adverse events (2 patients [3.7%]). No deaths were reported.
Dr. Necchi concluded his presentation of the TAR-200 monotherapy cohort of patients with CIS +/- papillary disease from SunRISe-1:
- Overall, centrally-assessed complete response rate with TAR-200 monotherapy was 76.7% in BCG-unresponsive HR NMIBC
- TAR-200 provides sustained and durable response
- 91% of responses (21 of 23) are ongoing, with a median follow-up in responders of 48 weeks
- 6 patients had a duration of response ≥12 months (all ongoing)
- Complete response rate was concordant between central and investigator assessments
- As of the clinical cutoff, no responders underwent radical cystectomy
- TAR-200 was well tolerated, mainly low grade 1 or 2, with manageable urinary symptoms
- TAR-200-related serious AEs, grade ≥3 AEs, and discontinuations were infrequent
- Efficacy and safety data from SunRISe-1 support the ongoing investigation of TAR-200 in patients with BCG-unresponsive HR NMIBC
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.
- Black PC, Tangen CM, Singh P, et al. Phase 2 Trial of Atezolizumab in Bacillus Calmette-Guérin-unresponsive High-risk Non-muscle-invasive Bladder Cancer: SWOG S1605. Eur Urol. 2023.
- Boorjian SA, Alemozaffar M, Konety BR, et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: A single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2020 Nov 27:S1470-2045(20)30540-4.


