(UroToday.com) The 2023 ESMO annual meeting included a session on novel targets, therapies, and toxicities in genitourinary cancers, featuring a presentation by Dr. Andrea Necchi discussing screening new agents in “window of opportunity” neoadjuvant trials. Dr. Necchi notes that the clinical model of bladder cancer is the ideal landscape for neoadjuvant treatment/trials:
Importantly, 50% of patients with muscle-invasive bladder cancer are unfit for neoadjuvant chemotherapy and a proportion are unfit for radical cystectomy. Because of this generally poor performance status, a 12-week delay from diagnosis to radical cystectomy or from the end of neoadjuvant chemotherapy and proceeding to radical cystectomy is safe. However, even after radical cystectomy, the 5-year mortality rate of patients with muscle-invasive bladder cancer is 50% - 70%. Dr. Necchi presented a case of a 52 year old female who presented in 2018 with cT3aN0M0 urothelial carcinoma and refused cisplatin-based chemotherapy. She was subsequently enrolled in the PURE-01 trial and received pembrolizumab x 3 cycles prior to cystectomy. He notes there are many challenges with neoadjuvant therapy, which include:
- Optimal clinical staging
- Tumor biomarkers of therapeutic activity
- Type of perioperative therapy
- Clinical and pathologic response surrogacy for overall survival
As follows are the neoadjuvant IO or IO-IO therapy trials for muscle invasive bladder cancer, with pT0N0 rates ranging from 18%-46%: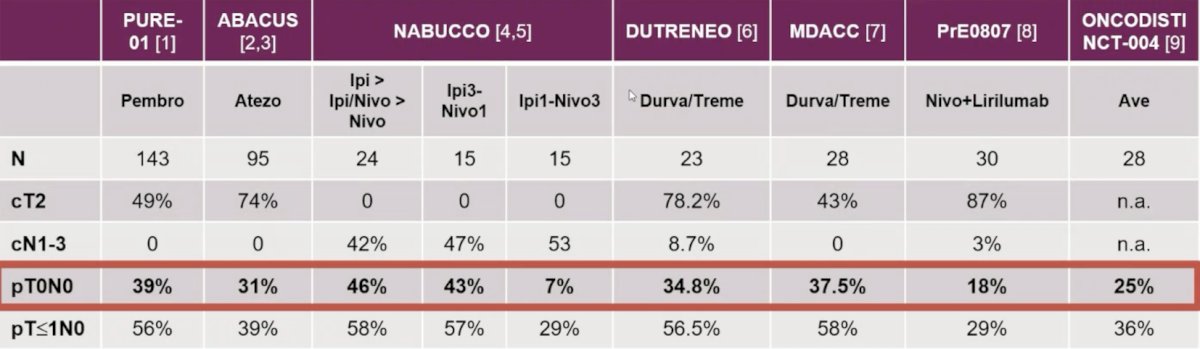
In the old chemotherapy paradigm in the neoadjuvant bladder cancer trials, pCR has been associated with improved overall survival: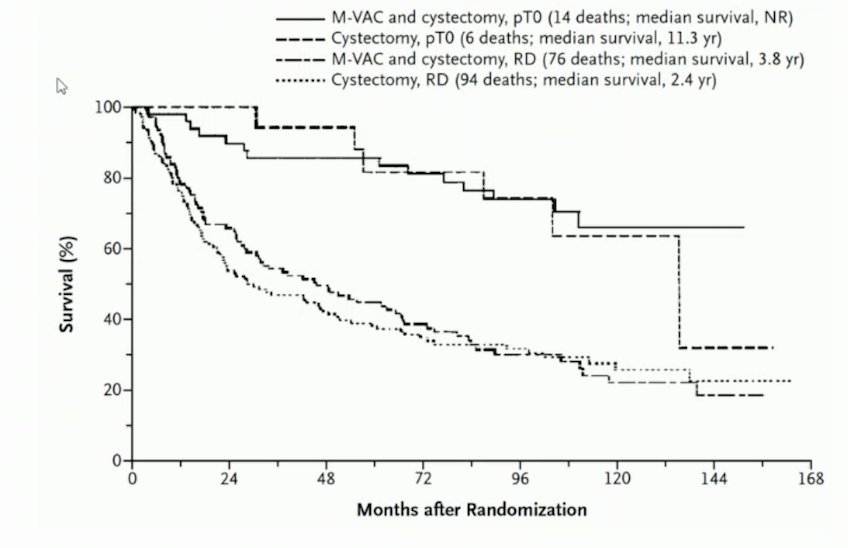
Based on data from NABUCCO,2 ABACUS,3 and PURE-01,4 several immunotherapy courses appear likely to offer disease control: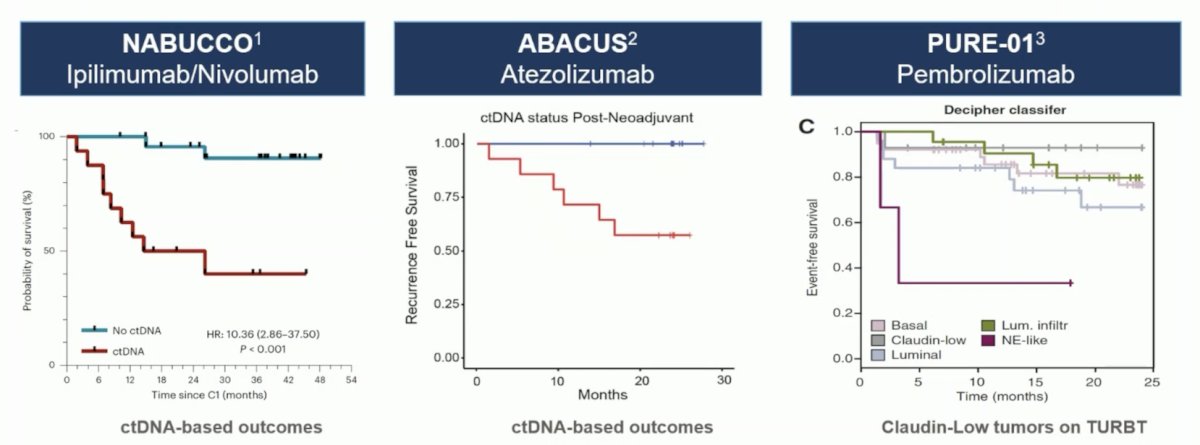
Antibody-drug conjugates are also attractive options as neoadjuvant therapies in muscle invasive bladder cancer. We have seen encouraging data for enfortumab vedotin in EV-103 and sacituzumab govitecan in SURE-01. However, each of these trials/agents have had small, but not insignificant grade 5 adverse event rates:
The following outlines the current active phase 3 trials of enfortumab vedotin in muscle invasive bladder cancer:
Dr. Necchi then discussed the TAR-200-103 trial which enrolled 26 patients that completed 4 TAR-200 dosing cycles and then underwent biopsy/pathological assessment: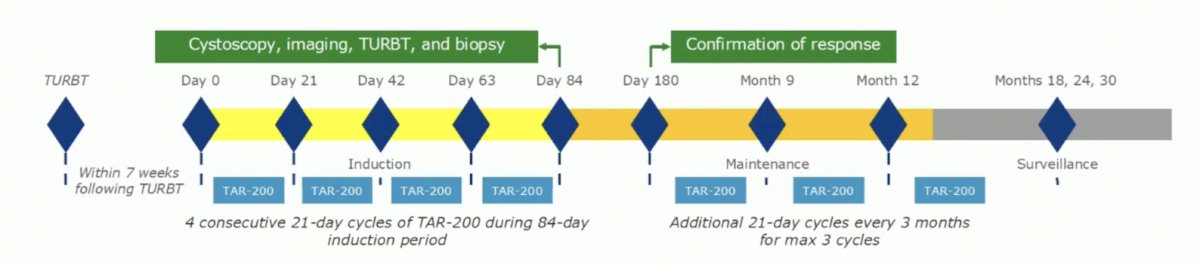
The PFS rate in responders at 12 months was 67.7% and median OS was 27.3 months with 8 patients still receiving ongoing surveillance at the time of data-cutoff. Importantly, the Sunrise-4 trial is underway. Another potential target is GDF-15, a potent tumor-derived immunosuppressant that is stress-induced and part of the TGF-B family. The GDFATHER-1 trial is assessing visugromab as a GDF-15 neutralizing antibody, which has previously shown to have tumor regression rates for DL 3-5/4-5 in a phase 1 trial of 22%-26%. The phase 2 trial development commenced February 1, 2022, and is ongoing.
The immune checkpoints LAG-3 and TIGIT have been shown to contribute to treatment resistance in many cancers and have been shown to be expressed in bladder cancer. Because inhibition of TIGIT and LAG-3 may enhance the efficacy of pembrolizumab, cohort C of KEYNOTE-057 will evaluate the efficacy and safety of coformulations of pembrolizumab with the TIGIT inhibitor vibostolimab or with the LAG-3 inhibitor favezelimab in patients with high risk BCG-unresponsive NMIBC with CIS +/- papillary tumors: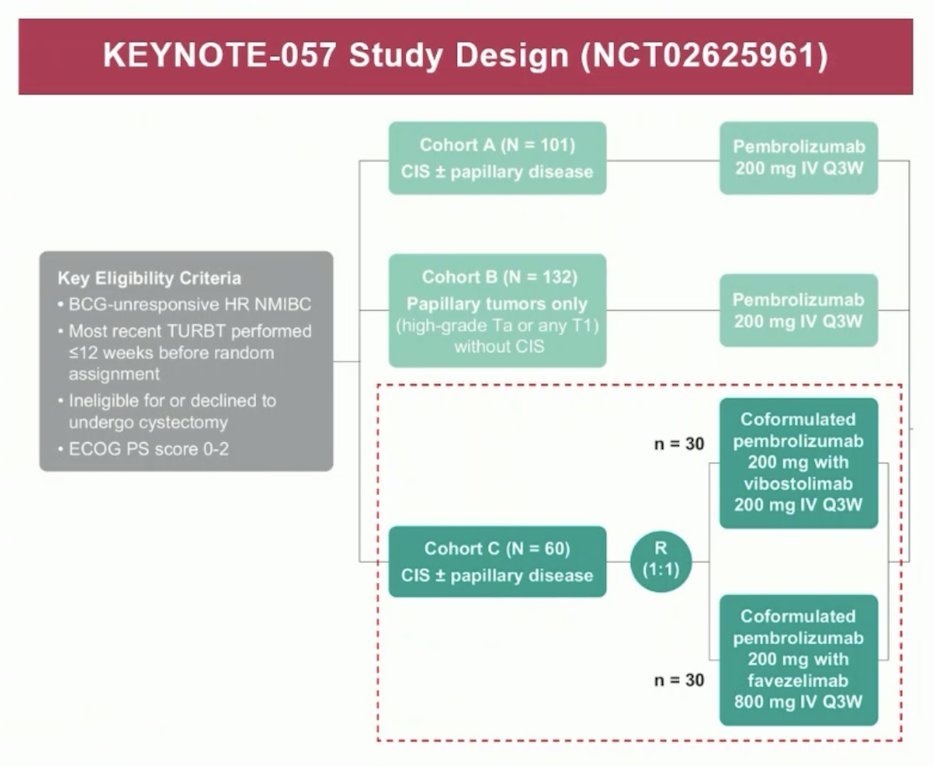
Dr. Necchi notes that it is important to ensure the safest neoadjuvant vs adjuvant therapy approaches given that safety of perioperative novel therapies is of utmost importance in a potentially curative setting like in muscle-invasive bladder cancer. Current tools that are used to qualify and grade treatment related adverse events may not necessarily fit the peculiar needs of perioperative therapies (ie. monitoring postsurgical toxicities). With regards to what is a safety event, there are many unanswered questions:
- What is the maximum acceptable time between the end of neoadjuvant therapy and radical cystectomy?
- Should we count any logistical issues preventing therapy administration as safety events?
- What is the maximum acceptable time between the radical cystectomy and the start of adjuvant therapy?
- What is the acceptable threshold of grade 3-5 treatment related adverse events at a trial level?
Dr. Necchi offered the following practical tips for the urology/oncology practice in 2023: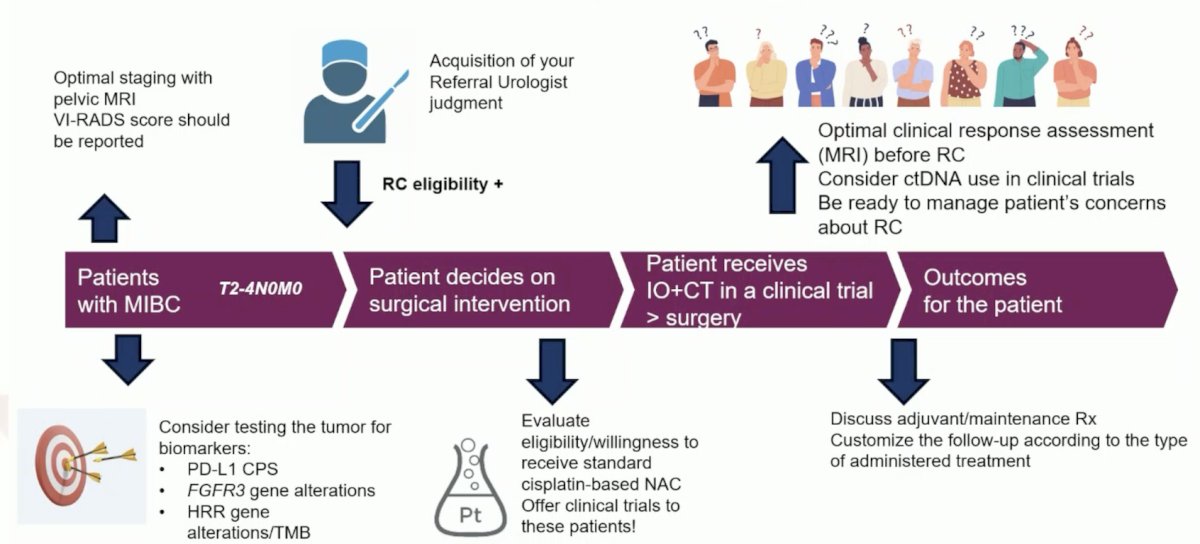
With regards to bladder preservation using systemic therapy, Dr. Necchi favors a response-based flexibility. Indeed, cystectomy and radical radiotherapy are associated with morbidity and quality of life implications. Some patients have a remarkable response to neoadjuvant systemic therapy and there are favorable data on outcomes with TURBT + chemotherapy in select patients. So, can deep responders to systemic therapy keep their bladders intact, and can a neoadjuvant window of opportunity result in a bladder-sparing opportunity? Several trials are envisioning new avenues after systemic therapy and new concepts for consolidation therapy: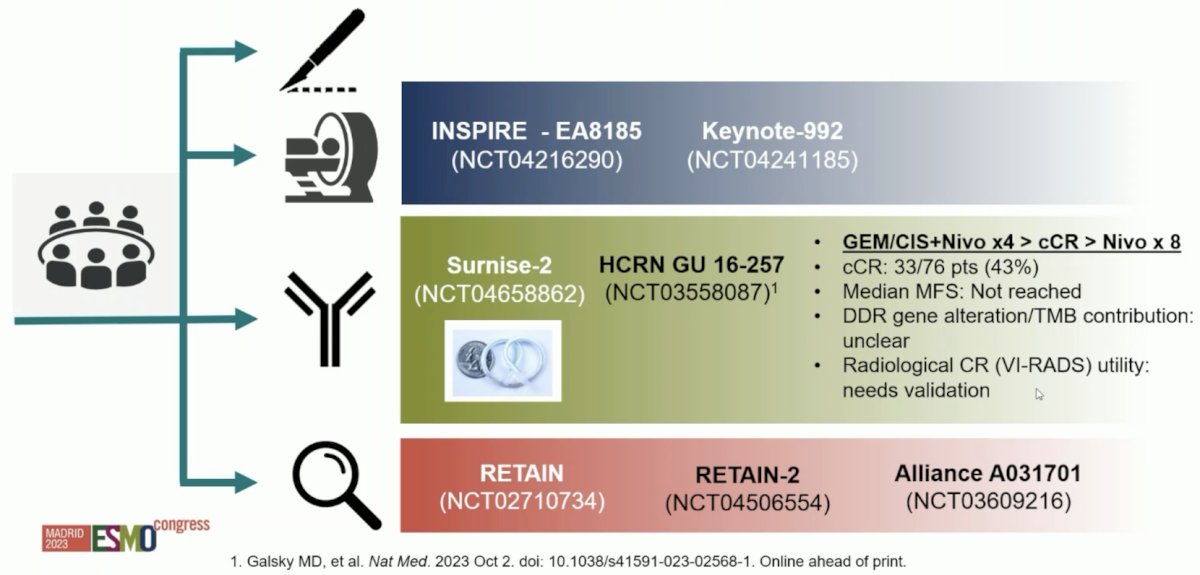
Dr. Necchi concluded his presentation discussing screening new agents in “window of opportunity” neoadjuvant trials with the following take-home points:
- Neoadjuvant trials provide a one of a kind opportunity for accelerated drug approvals in oncology
- Testing the most active new drugs in curable settings can provide larger magnitude gains in survival
- Safety remains a concern requiring continuous patient and physician education – the safety/efficacy threshold remains uncertain in the perioperative setting
- Trials of patient allocation for bladder preservation based on response are an intelligent way of addressing patient concerns about radical cystectomy, but we need harmonization of design and outcomes
- There is a need for consensus on what constitutes cure and curative intent in muscle-invasive bladder cancer given the emergence of novel, non-surgical therapy options to support adequate clinical trial designs and health care decision making
Presented by: Andrea Necchi, MD, San Raffaele, Milan, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
- van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020 Dec;26(12):1839-1844.
- Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019 Nov;25(11):1706-1714.
- Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients with Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. J Clin Oncol 2018 Dec 1;36(34):3353-3360.


