(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a neoadjuvant and adjuvant therapy in genitourinary cancers session. Dr. Maria De Santis delivered a state-of-the-art lecture discussing the current state of adjuvant therapy for urothelial carcinoma.
Dr. De Santis began by emphasizing that the general goal of adjuvant therapy is to increase the cure rate, without exposing patients to life-changing side effects. For urothelial carcinoma, eligible options in this setting include chemotherapy, immunotherapy, and radiotherapy. Efficacy outcomes of particular interest in this cohort include overall survival (OS), disease-free survival (DFS), local control, and metastasis-free survival (MFS).
Why does adjuvant therapy matter in these patients? The fact remains that 5-year survival for localized and locoregional bladder cancer remains low, with patients with completely resected, high-risk urothelial carcinoma having a 5-year survival rate of only 60%. This is particularly worse for higher risk cohorts of patients with ≥pT2 or pN+ disease following neoadjuvant chemotherapy.

Is there any role for adjuvant chemotherapy? Currently, the ESMO guidelines recommend neoadjuvant chemotherapy with ddMVAC or gem-cis based on a known 5 – 8% OS benefit in this setting.1 These guidelines note that there is ‘weak evidence’ for the use of adjuvant cisplatin-based chemotherapy in patients who did not receive neoadjuvant therapy.

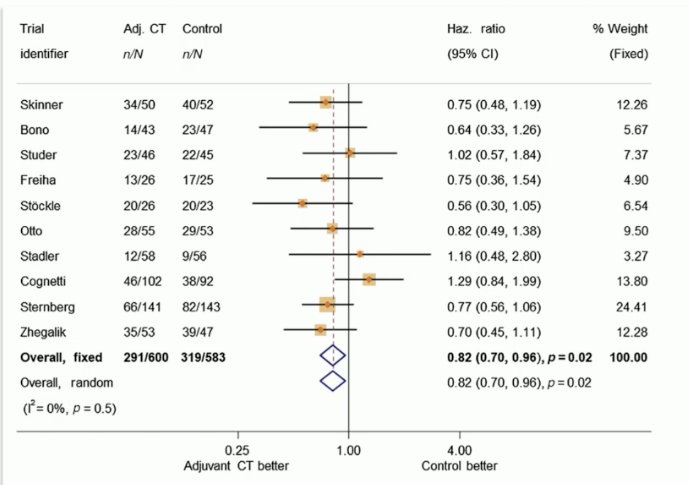
Summarized below is the current evidence for chemotherapy in the muscle-invasive bladder cancer (MIBC) adjuvant setting. The only ‘positive’ trial in this space is the EORTC 30994 trial led by Dr. Sternberg. This trial randomized 24 patients with pT3-4 or pN+ disease to either chemotherapy (gem-cis, MVAC, or ddMVAC) versus a control arm of observation followed by chemotherapy at relapse. PFS was significantly improved from 32% to 48% (HR: 0.54, p<0.001). While median OS was prolonged by about two years (6.7 versus 4.6 years), the 5-year OS was non-significantly different (54% versus 48%, p=0.13).2

In 2022, the Advanced Bladder Cancer (ABC) group conducted a meta-analysis of the 10 trials in this space, including the 7 that were closed prior to completion due to either futility or slow accrual, and demonstrated that, in the aggregate, there is an overall survival benefit to adjuvant cisplatin-based chemotherapy (HR: 0.82, 95% CI: 0.70 – 096, p=0.02).2

The unadjusted 5-year OS benefit with adjuvant cisplatin-based chemotherapy appears to be 6% (56% versus 50%), with the OS benefit following adjustment for age, sex, pT stage, and pN stage estimated at 9%. Toxicity data was inconsistently reported but overall appears to be consistent with the known toxicity profile of this combination.
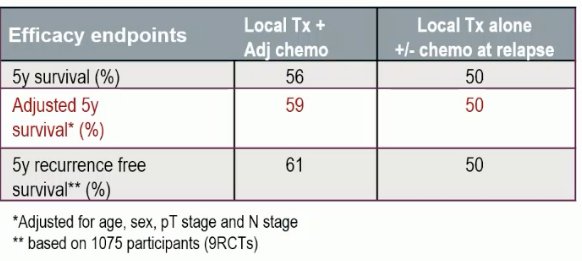
Based on this evidence, the EAU currently recommends offering adjuvant cisplatin-based combination chemotherapy to patients with pT3/4 and/or pN+ disease if no neoadjuvant chemotherapy has been given.

What about immunotherapy in the adjuvant setting? To date, there have been three trials of adjuvant immunotherapy in high risk, muscle invasive upper or lower urothelial tract malignancies published/presented in this space: AMBASSADOR, CheckMate 274, and IMvigor 010.4-6 Two of these trials (AMBASSADOR [via a press release October 2023] and CheckMate 274) have demonstrated DFS benefits, whereas one (IMvigor 010) was negative for its primary endpoint of DFS.

In the CheckMate 274 trial, adjuvant nivolumab improved DFS in the intention to treat the population from 11 to 21 months (HR: 0.70, p<0.001). This effect was even more pronounced in the PD-L1 high population (HR: 0.53, p<0.001). Conversely, atezolizumab failed to show a DFS benefit in either population.
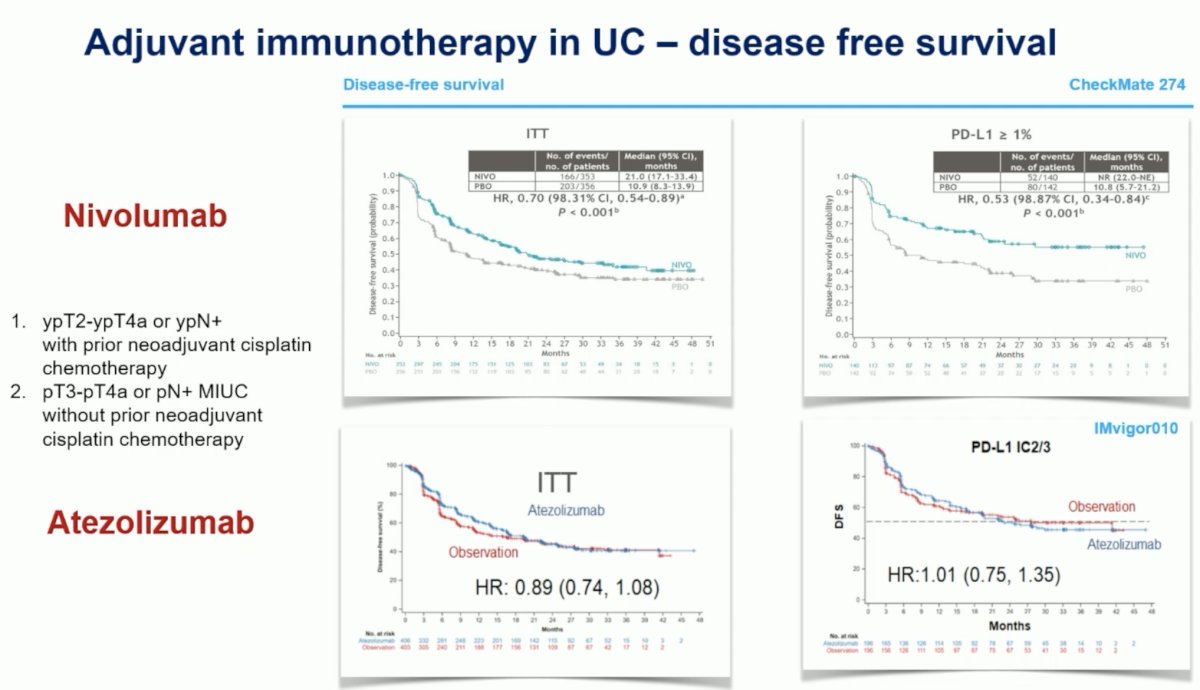
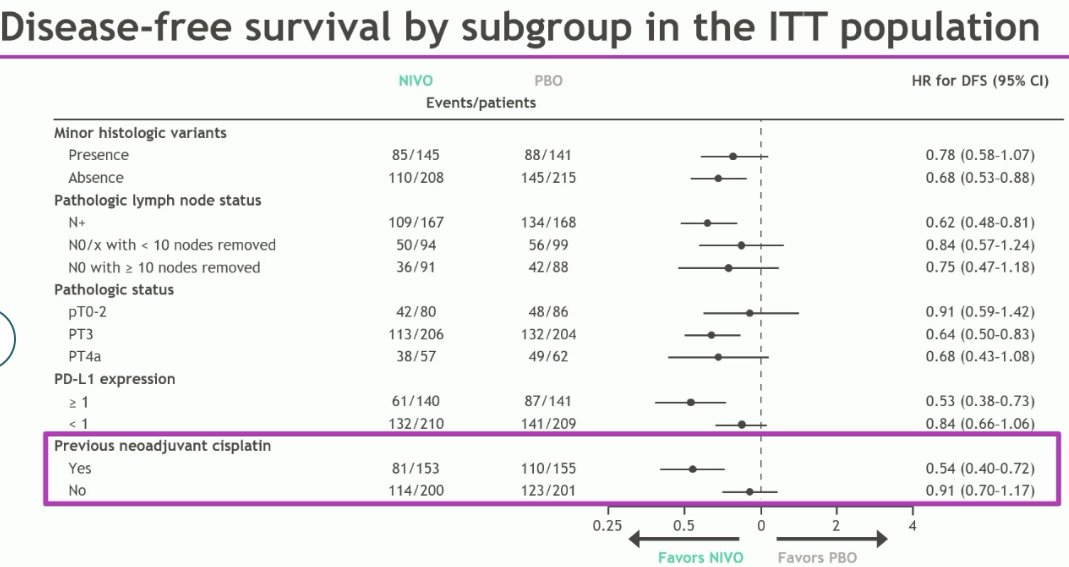
Subgroup analysis of the extended follow-up of CheckMate 274 presented at ASCO GU 2023 demonstrates that the benefit of nivolumab is considerably lower in the PD-L1 expression low patients (HR: 0.84, 95% CI: 0.66 – 1.06). Similarly, patients who did not receive (or potentially require) neoadjuvant chemotherapy derived a significantly lower DFS benefit compared to those who did (HR: 0.91 versus 0.54).
From a safety standpoint, the most common treatment-related adverse events (TRAEs) with nivolumab were pruritis (23%), fatigue (17%), and diarrhea (17%). Grade 3+ TRAEs occurred in 18% of patients, and overall, 7% led to treatment discontinuation (16% with atezolizumab in IMvigor 010).

In light of these results, the EAU guidelines currently ‘weakly recommend offering adjuvant nivolumab to selected patients with pT3-4 and/or pN+ disease not eligible for, or who decline, adjuvant cisplatin-based chemotherapy.

Conversely, the ESMO guidelines currently do not recommend adjuvant immunotherapy due to conflicting results from trials, with no available OS data to yet, despite nivolumab being European Medical Agency (EMA) approved for MIBC patients with tumor cell PD-L1 expression ≥1%, who are at high risk of recurrence following surgery.
But do we need to show an OS benefit in adjuvant MIBC trials, or is DFS a sufficient surrogate endpoint? A meta-analysis suggests that while distant metastasis-free survival is a valid, reliable surrogate, DFS specifically is more variable with regards to reliably predicting OS outcomes, particularly at the trial level (R2=0.69).

What about adding adjuvant radiotherapy to adjuvant chemotherapy, particularly given there is a 30% local-regional failure after radical cystectomy in pT3-4 MIBC patients? In a randomized phase II trial of 120 patients with MIBC, Zaghloul et al. evaluated adjuvant sequential chemohormonal radiotherapy versus adjuvant chemotherapy in patients with locally advanced disease and negative margins. They demonstrated that the addition of radiotherapy to adjuvant chemotherapy improved 2-year local relapse-free survival from 69% to 96%.7 Based on these results, the EAU currently offers a weak recommendation for offering adjuvant radiotherapy in addition to chemotherapy following radical cystectomy, based on pathologic risk (pT3b-4, pN+, or positive margins).
What about patient preferences in this setting? An observational, cross-sectional of 207 MIBC patients demonstrated that patients would choose adjuvant treatment if it prolonged OS in 91.2% of cases. Prolonging OS from 25 to 78 months was most important, followed by reducing serious side effects and risks.

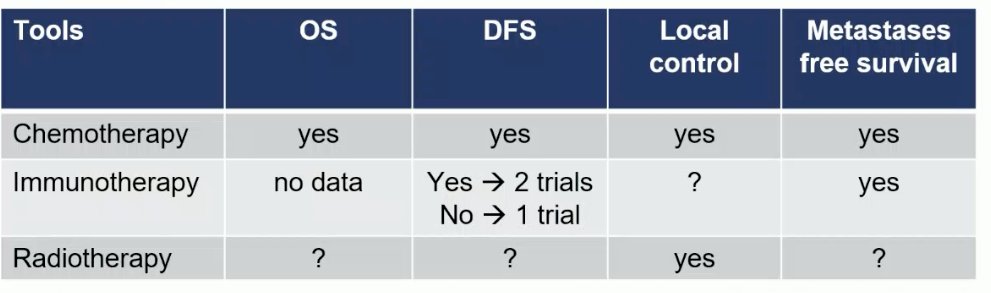
To conclude, Dr. De Santis concluded the following regarding the currently available evidence in the urothelial carcinoma adjuvant space:
- Cisplatin-based chemotherapy prolongs OS, DFS, local control, and MFS
- Immunotherapy improves DFS in 2/3 trials and MFS, but not OS to date
- Radiotherapy adjuvantly improves local control when co-administered with chemotherapy, but other survival/efficacy outcomes are pending
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349(9):859-866.
- Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomized phase 3 trial. Lancet Oncol. 2015 Jan;16(1)76-86.
- Advanced Bladder Cancer (ABC) Meta-analysis Collaborators Group. Adjuvant Chemotherapy for Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis of Individual Participant Data from Randomised Controlled Trials. Eur Urol. 2022; 81(1):50-61.
- Apolo AB, Rosenberg JE, Kim WY, et al. Alliance A031501: Phase III randomized adjuvant study of MK-3475 (pembrolizumab) in muscle-invasive and locally advanced urothelial carcinoma (MIBC) (AMBASSADOR) versus observation. J Clin Oncol. 2019;37(Suppl 7).
- Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021 Jun 3;384(22):2102-2114.
- Bellmunt J, Hussain M, Gschwend JE, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): A multicentre, open-label, randomized, phase 3 trial. Lancet Oncol. 2021 Apr;22(4):525-537.
- Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant Sandwich Chemotherapy Plus Radiotherapy vs Adjuvant Chemotherapy Alone for Locally Advanced Bladder Cancer After Radical Cystectomy. JAMA Surg. 2018;153(1):e174591.


