(UroToday.com) The 2023 ESMO annual meeting included a session on optimizing overall survival in advanced renal cancer, featuring a presentation by Dr. Axel Bex discussing the role of localized therapies for metastatic RCC. The rationale for localized treatment in metastatic RCC is that in localized disease in which complete resection is achievable, there is a possibility of cure, an improvement in disease free survival, progression free survival, and overall survival, and a disease-free interval with delay or discontinuation of systemic therapy. Dr. Bex highlighted the following entities and nomenclature for local therapeutic options:
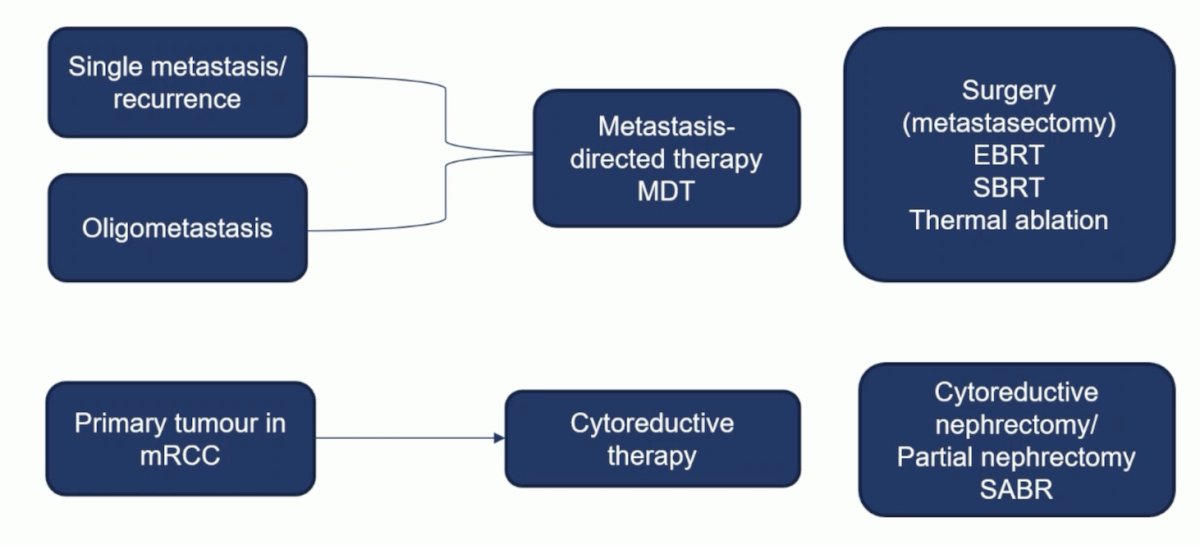
When discussing oligometastasis, it is important that we are speaking the same language. Quantitative (number of metastases) and developmental (time) characteristics are most commonly used:
- Is the metastasis de novo?
- Is it synchronous or metachronous?
- Did it occur under treatment?
- Do two or more organ sites qualify for oligometastatic disease?
As follows are the 5-year survival rates following complete resection of solitary or oligometastatic RCC:
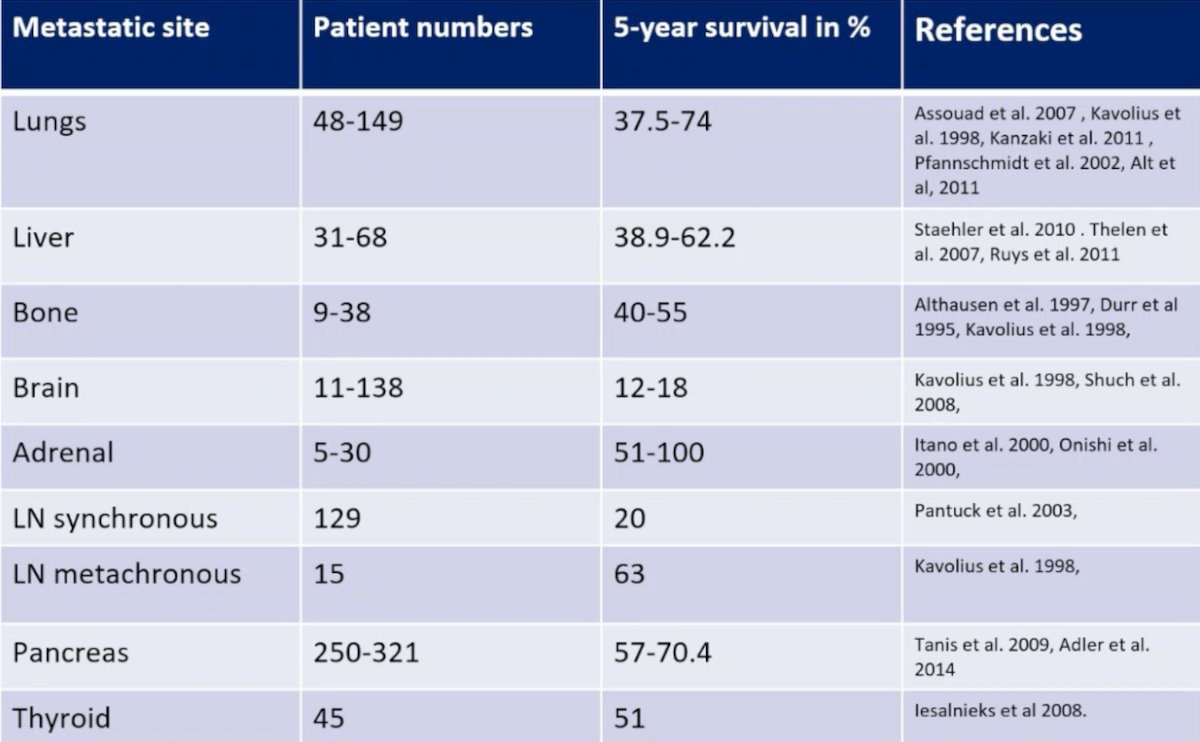
So, is ‘cure’ with localized therapy alone a realistic goal? Dr. Bex used the example of an isolated lymph node metastasectomy. This data varies: in one cohort, after resection of lymph node metastases (1-3 lymph nodes), the median time to development of distant metastases was only 4.2 months. In another cohort of RCC patients with resection of an isolated lymph node, the median progression free survival was 19.5 months, 3-year CSS was 75.8%, and 5-year CSS was 73.6%. Dr. Bex notes that there is a retrospective bias associated with metastasectomy series’ as highlighted in the following figure:

In a study from Dr. Bex’s group, they assessed local treatment of recurrent RCC and the affect on survival across different groups using the RECUR multicenter European registry.1 Among 3,039 patients with localized RCC treated with curative intent, 505 presented with recurrence, including 176 with resectable disease. Among these patients, 97 underwent local treatment of recurrence and 79 no local treatment of recurrence. The median OS was 70.3 months (95% CI 58-82.6) versus 27.4 months (95% CI 23.6-31.15) in the local treatment of recurrence versus no-local treatment of recurrence group (p < 0.001). The local treatment of recurrence effect on survival was consistent across risk groups: OS hazard ratios for high, intermediate, and low risks were 0.36 (95% CI 0.2-0.64), 0.27 (95% CI 0.11-0.65), and 0.26 (95% CI 0.08-0.8), respectively:

Unfortunately, we do not know what the true impact of metastasectomy is on survival, as we have no randomized controlled trials in metastatic RCC to answer this question. What we do know is that selection is key, and that there are many factors contributing to the outcome: performance, site-specific factors, and development over time. Furthermore, the recurrence free interval is a reflection of tumor biology – ie. disease-free interval of > 2 years. As such, Dr. Bex notes that we can know the position of the metastasis, but not the momentum of the metastatic progression. Additionally, it is important to note that ‘surgical’ metastasectomy comes at a price. In a study assessing in-hospital complications among 1,102 patients undergoing metastasectomy from 45,279 metastatic RCC patients in the National Inpatient Sample database (2000-2011), the major complication rate (Clavien III-IV) was 25.1%:2

Other local treatment modalities are being evaluated, including stereotactic body radiotherapy:
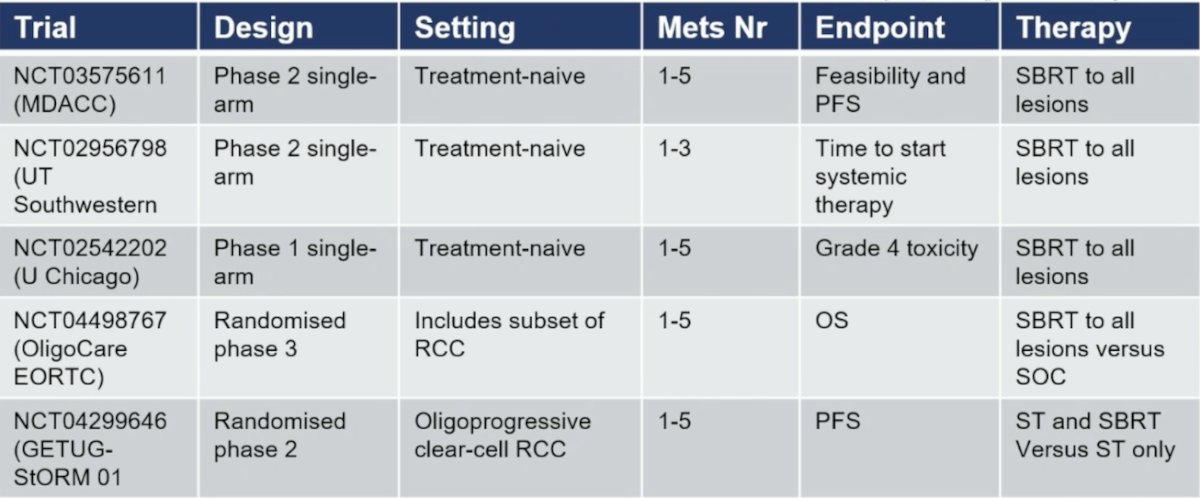
There are several small single-arm trials investigating stereotactic body radiotherapy in oligoprogression, with median time to changing therapy ranging from 11.1-12.6 months. Also, trials are assessing stereotactic body radiotherapy in combination with immune checkpoint inhibition, including the RAPPORT phase 1/2 trial of 30 patients using stereotactic body radiotherapy to all metastases followed by 6 months of pembrolizumab. This combination has a 1-year OS rate of 90% and 2-year OS rate of 74%. It is important to note that the KEYNOTE-564 trial3 includes a small cohort of patients that are M1 resected to no evidence of disease who then received 1 year of adjuvant pembrolizumab. Among, these patients who received pembrolizumab, they did very well with a DFS HR of 0.28, 95% CI 0.12-0.66 compared to observation alone:

Alternatively, in the real world setting, surveillance of metastases is frequent (32%) and a safe alternative to immediate systemic therapy, with a median time to therapy of 16 months. Ultimately, clinical trials and appropriate clinical trial design is key. Dr. Bex notes that the optimal trial of local therapy versus no local therapy of metastases would look as follows:
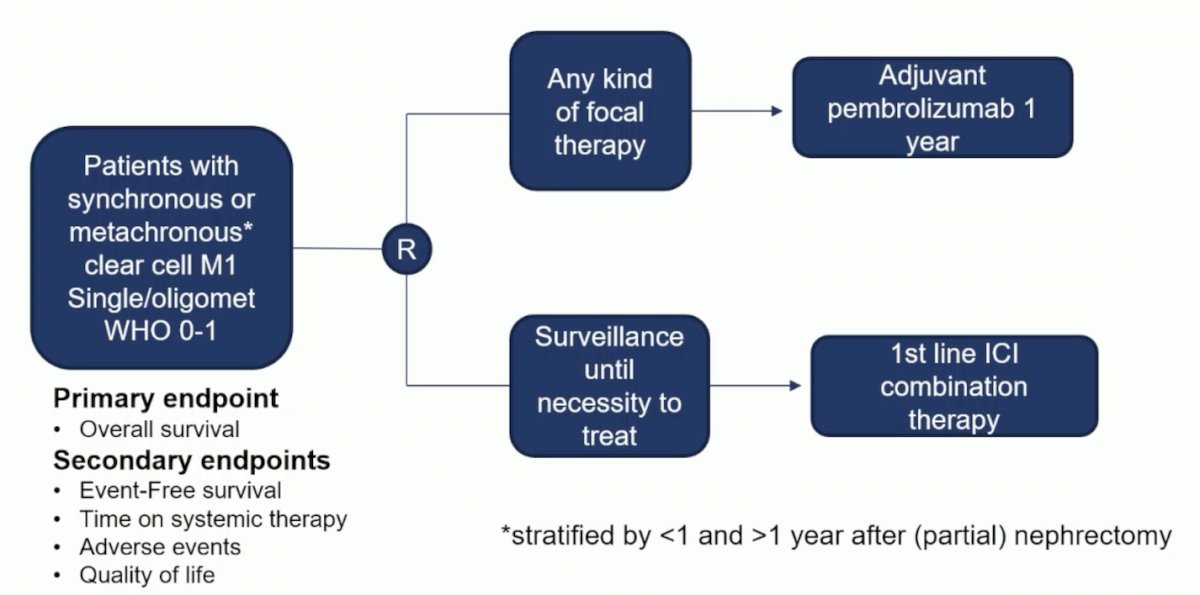
Dr. Bex notes that indications for deferred cytoreductive nephrectomy should generally be discussed at multidisciplinary tumor boards. Several potential indications for deferred cytoreductive nephrectomy are as follows:
- The patient develops a durable complete/near complete response at metastatic sites and can be rendered disease free by removal of the primary tumor
- The patient has developed durable response or stable disease at metastatic sties, but the primary tumor is progressing locally
Additional arguments for deferred cytoreductive nephrectomy may include:
- Complete pathological response in the primary tumor is rare and vital tumor may remain in patients with complete response at metastatic sites
- Removal of the primary tumor may abrogate rapidly metastasizing clones and potentially prolong survival
- Patients with complete response may potentially stop immune checkpoint inhibitor therapy after cytoreductive nephrectomy
- Currently, confirmation of pathological complete response of the primary tumor requires full histopathological examination of the specimen
Two key trials of local therapy of the primary tumor and immune checkpoint inhibitor therapy incorporating deferred cytoreductive nephrectomy with a primary endpoint of OS include the NORDIC-SUN trial and the PROBE SWOG S1931 trial:
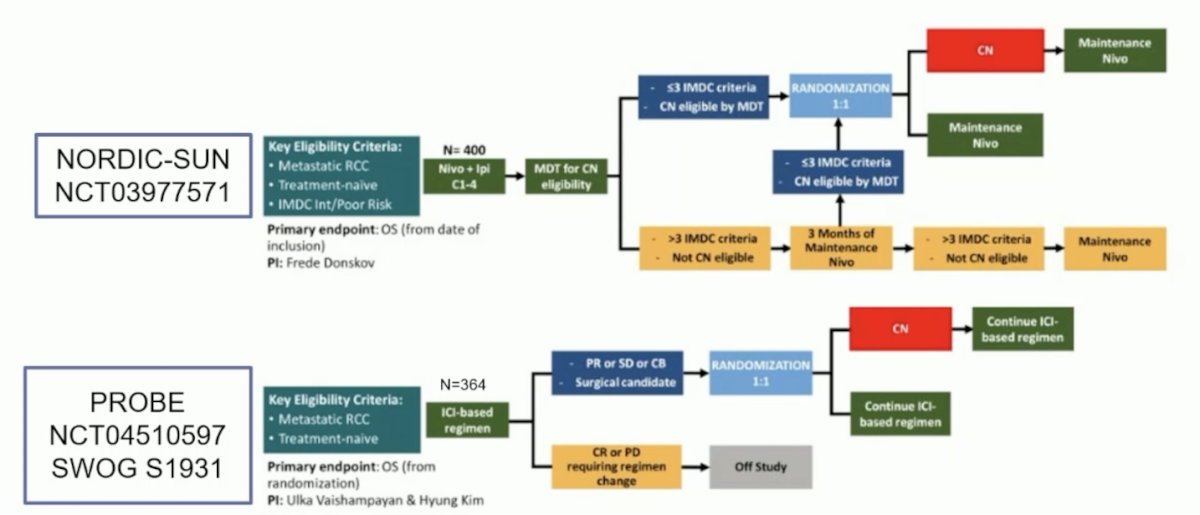
Additionally, two trials assessing local therapy of the primary tumor and immune checkpoint inhibitor therapy with a primary endpoint of PFS include the SAMURAI trial and the CYTOSHRINK trial:
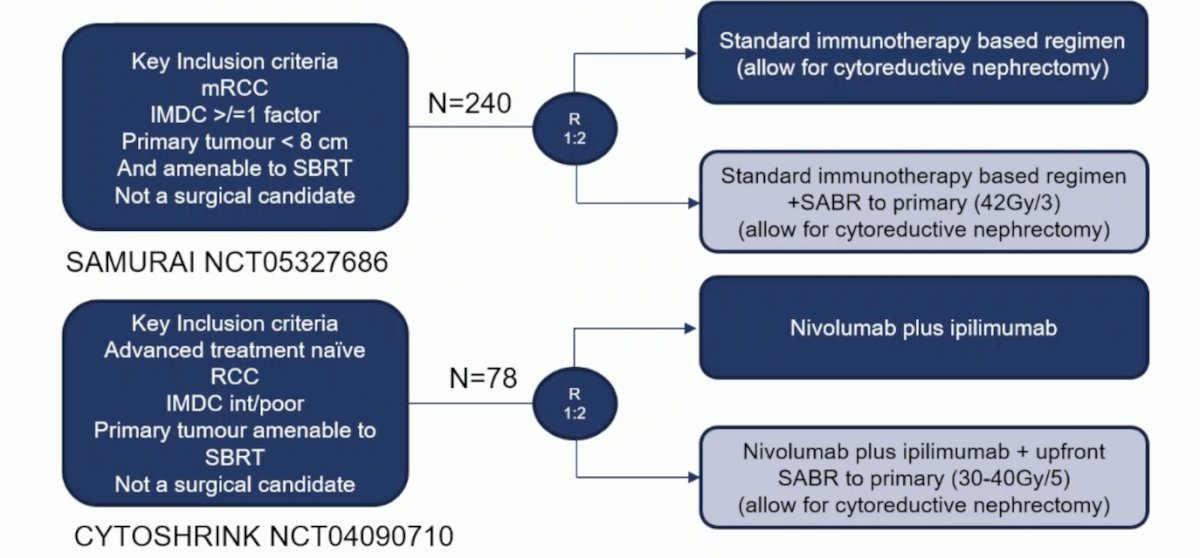
Finally, Dr. Bex highlighted the randomized trial landscape for local treatment of metastatic RCC, with specific objectives to help categorize the trials:
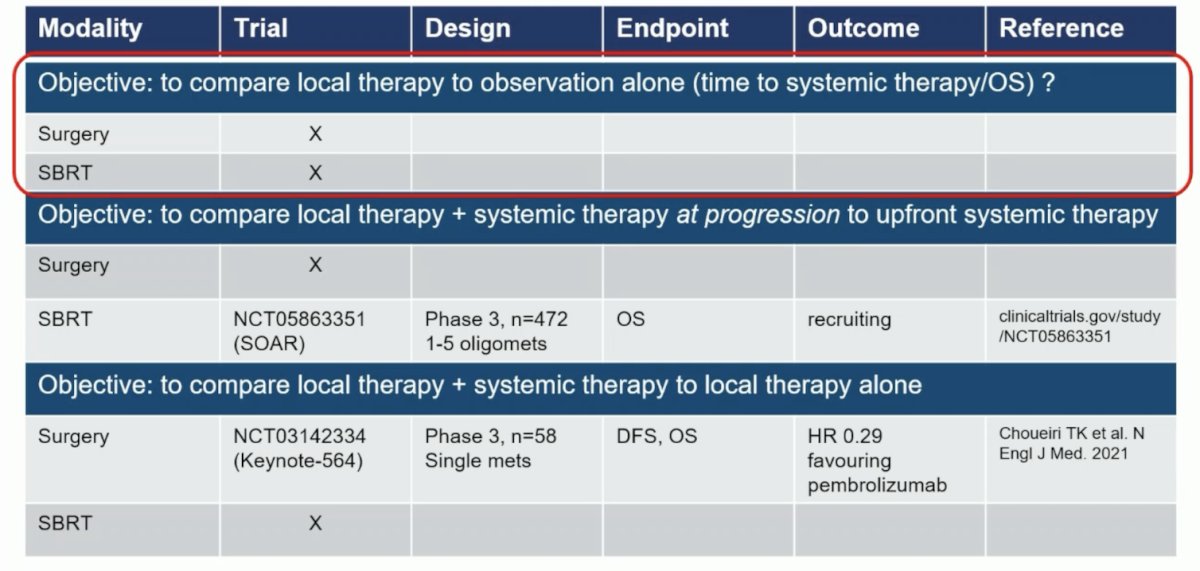
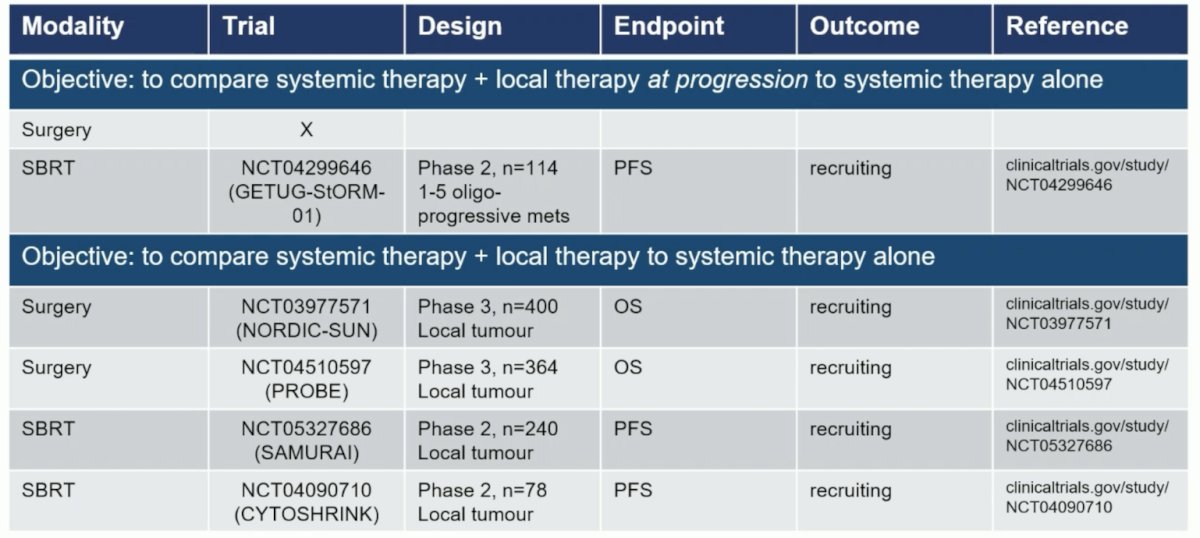
Dr. Bex concluded his presentation by discussing the role of localized therapies for metastatic RCC with the following take-home points:
- The role of localized therapy in metastatic RCC is ill-defined
- The evidence base has largely emerged from retrospective studies of metastasectomies using surgery by default but remains poor due to an absence of RCT data
- Prolonged disease-free intervals with delay or discontinuation of systemic therapy are more realistic objectives than cure
- High recurrence rates and surgical adverse events following metastasectomy are not inconsequential, thus minimally invasive approaches, such as stereotactic body radiotherapy are gaining ground
- Oligometastatic disease comprises different clinical presentations and dynamics that need to be recognized in the design of trials
- Randomized controlled trials for different clinical settings are ongoing to improve the evidence base
Presented by: Axel Bex, MD, PhD, University College London, London, United Kingdom
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Marconi L, Kuusk T, Capitanio U, et al. Local treatment of recurrent renal cell carcinoma may have a significant survival effect across all risk-of-recurrence groups. Eur Urol Open Sci. 2022 Dec 15:47:65-72.
- Meyer CP, Sun M, Karam JA, et al. Complications after metastasectomy for renal cell carcinoma: A Population-based assessment. Eur Urol. 2017 Aug;72(2):171-174.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.


