(UroToday.com) The 2023 ESMO annual meeting included a trial in progress session on kidney cancer, featuring a presentation by Dr. Monty Pal discussing STELLAR-304, a randomized phase 3 study of zanzalintinib (XL092) and nivolumab in non-clear cell renal cell carcinoma.
Non-clear cell renal cell carcinomas (25% of all RCC) are a heterogeneous group of rare histologic subtypes, each with distinct presentation clinical course, and response to treatment. Given the rarity and heterogeneity of the disease, management of nonclear cell RCC is primarily based on results from phase 2 studies, subgroup analyses of RCC trials, meta-analyses, and treatments approved for clear cell RCC: There have been few dedicated randomized phase 3 studies in non-clear cell renal cell carcinoma, highlighting an urgent unmet need for effective therapies. Sunitinib is the only TKI to have shown a clinical benefit (vs everolimus) in a broad range of histologic subtypes of metastatic non-clear cell renal cell carcinoma (ASPEN trial).1 To date, no single agent or combination has shown a significant overall survival (OS) benefit over sunitinib in any non-clear cell renal cell carcinoma subtype. Zanzalintinib (XL092) is a novel TKI of VEGFR2, MET, AXL, and MER: 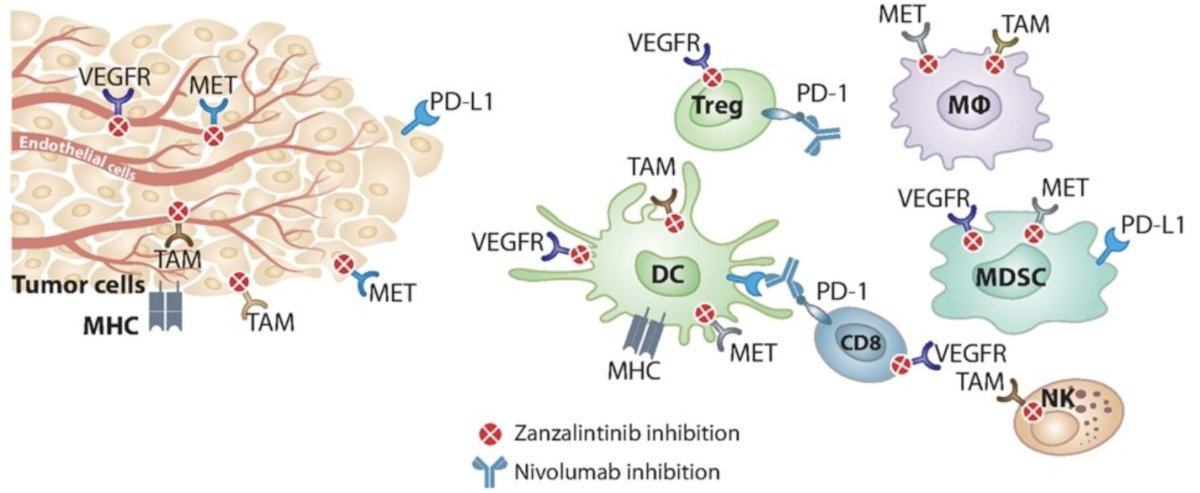
It has shown antitumor and immunomodulatory activity alone, and synergistic antitumor effect with PD1 inhibition, in animal models.2 Additionally, in a phase 1 study of metastatic solid tumors including RCC, zanzalintinib showed promising clinical activity and a manageable toxicity profile, alone and with an immune checkpoint inhibitor. STELLAR-304 was designed to assess the efficacy and safety of zanzalintinib + nivolumab versus sunitinib in first-line non-clear cell renal cell carcinoma (NCT05678673).
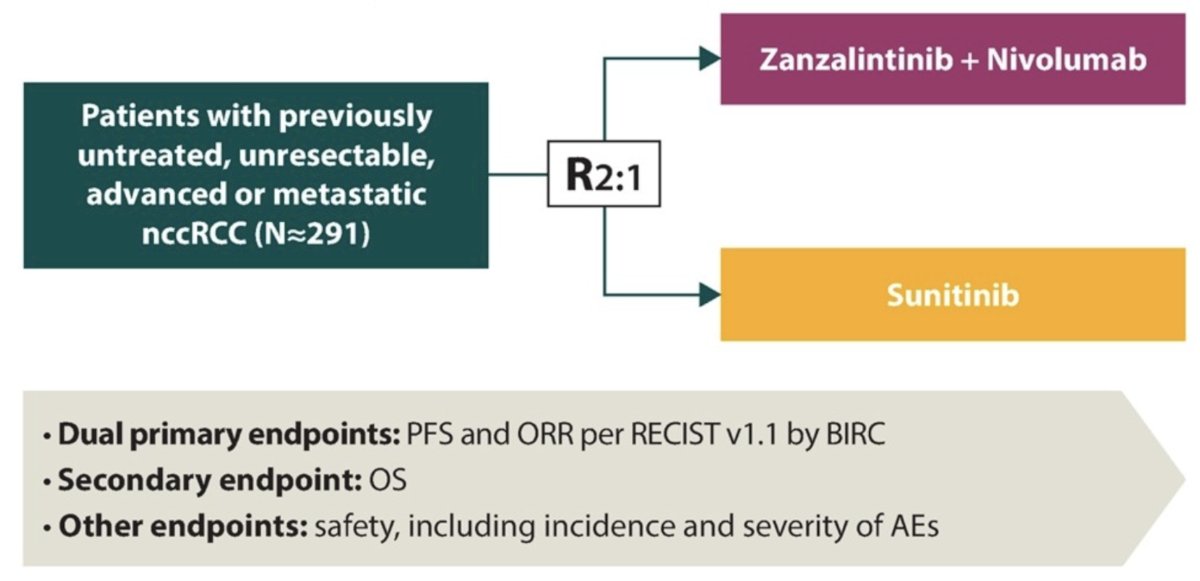
STELLAR-304 is a global, randomized, open-label, phase 3 study of adults with unresectable/advanced/metastatic non-clear cell renal cell carcinoma that is measurable per RECIST v1.1. The trial will enroll 291 patients with histologically confirmed, unresectable metastatic nonclear cell RCC who have not had prior systemic anticancer therapy for advanced disease. Eligible patients will be randomized 2:1 to receive zanzalintinib + nivolumab or sunitinib alone:
Patients with papillary, unclassified, and translocation-associated histologies (sarcomatoid features are allowed) will be eligible for enrollment. Chromophobe, renal medullary carcinoma, or pure collecting duct histology are excluded, and histologies are confirmed by central pathology review. Prior systemic anticancer therapy for advanced or metastatic non-clear cell renal cell carcinoma is not permitted, however,r one prior systemic adjuvant therapy, including immune checkpoint inhibitor and excluding sunitinib, is allowed for completely resected RCC if recurrence occurred ≥6 months after the last adjuvant therapy dose: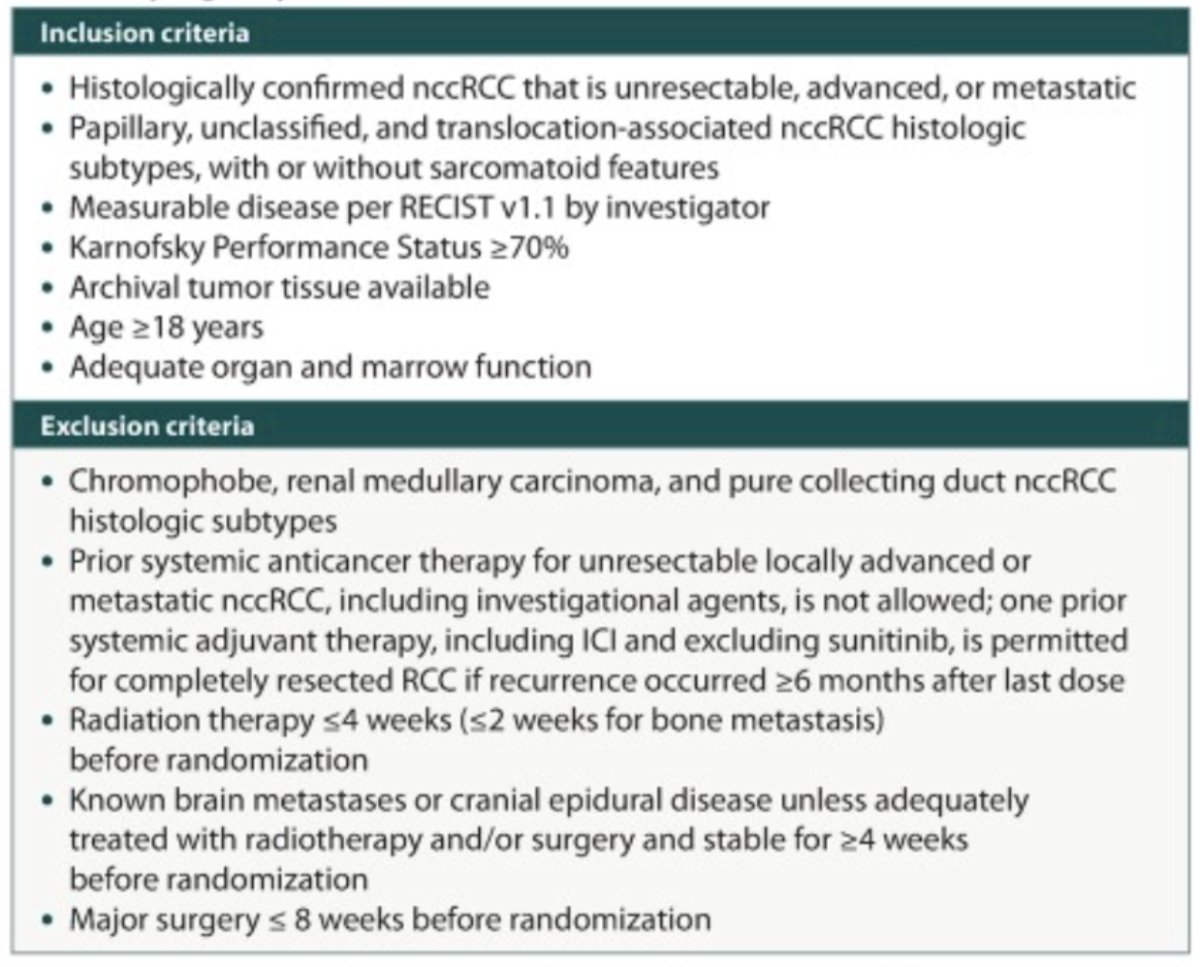
The primary endpoints are PFS and ORR per RECIST v1.1 by blinded independent radiology committee. The secondary endpoint is OS and safety will also be assessed. STELLAR-304 is currently recruiting patients in 29 countries in Europe, North, and South America, and the Asia-Pacific region: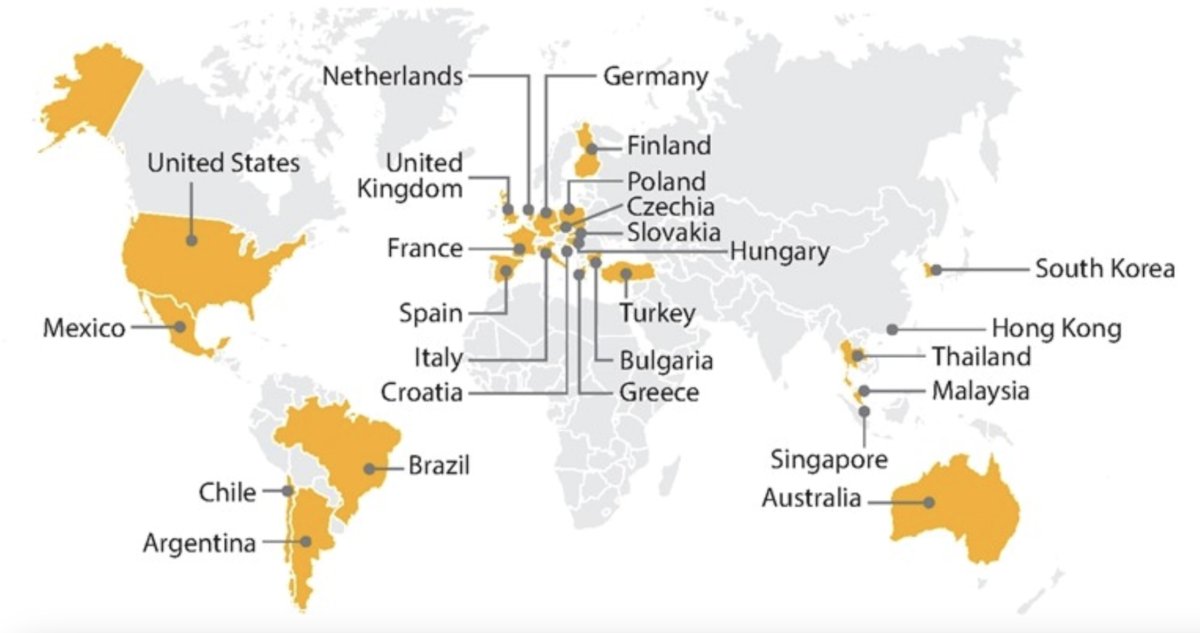
Presented by: Sumanta K. Pal, MD, FASCO, City of Hope Cancer Center, Duarte, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): A multicenter, open-label, randomized phase 2 trial. Lancet Oncol. 2016 Mar;17(3):378-388.
- Hsu J, Chong C, Serrill J, et al. Preclinical Characterization of XL092, a Novel Receptor Tyrosine Kinase Inhibitor of MET, VEGFR2, AXL, and MER. Mol Cancer Ther. 2023 Feb 1;22(2):179-191.


