(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a renal cancer abstracts poster session. Dr. Primo Lara presented the results of a subgroup analysis of the phase III SWOG S0931 (EVEREST) trial evaluating adjuvant everolimus in patients with completely resected very high-risk renal cell carcinoma (RCC) with clear cell histology.
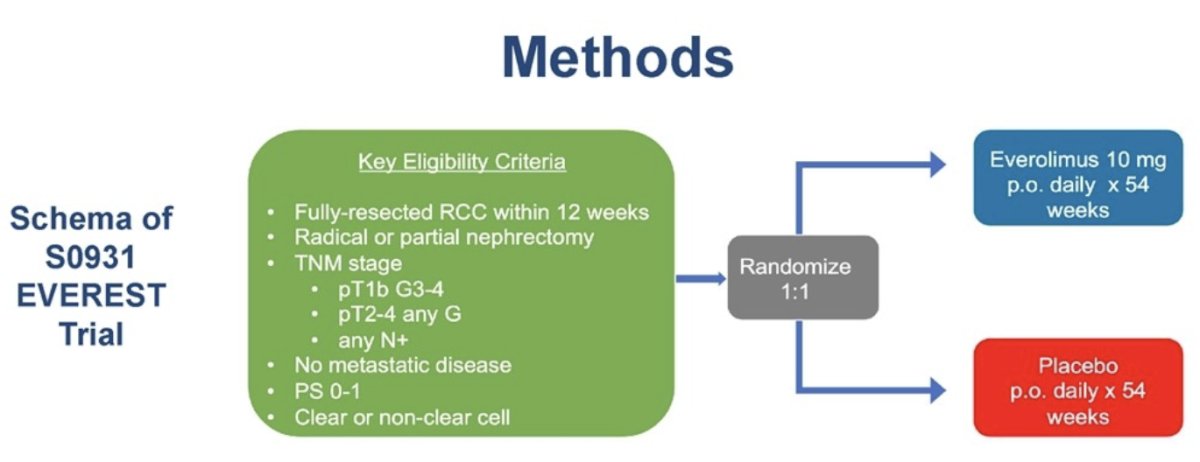
EVEREST is a randomized, double-blind, phase 3 trial that randomized patients (1:1) with histologically confirmed RCC (both clear cell and non-clear cell) who had undergone a full surgical resection and were at intermediate-high or very high risk of recurrence to either oral everolimus (10 mg daily) or placebo for 54 weeks. At a median follow-up of 76 months, recurrence-free survival was marginally improved with everolimus (5-years: 67% versus 63%, p=0.051). No overall survival benefits were observed for everolimus in this trial.1 In this study, Dr. Lara and colleague reported the results of a subgroup analysis of the very-high risk cohort of patients with clear cell RCC in EVEREST.
This cohort included post-nephrectomy RCC patients with any clear cell component and very-high risk disease, defined as any of:
- pT3a, Gr 3-4
- pT3b-c, any grade
- pT4, any grade
- pN+
Baseline patient characteristics, treatment received, and adverse event data were collected. Time-to-event analyses were performed using Cox regression modeling stratified by performance status.
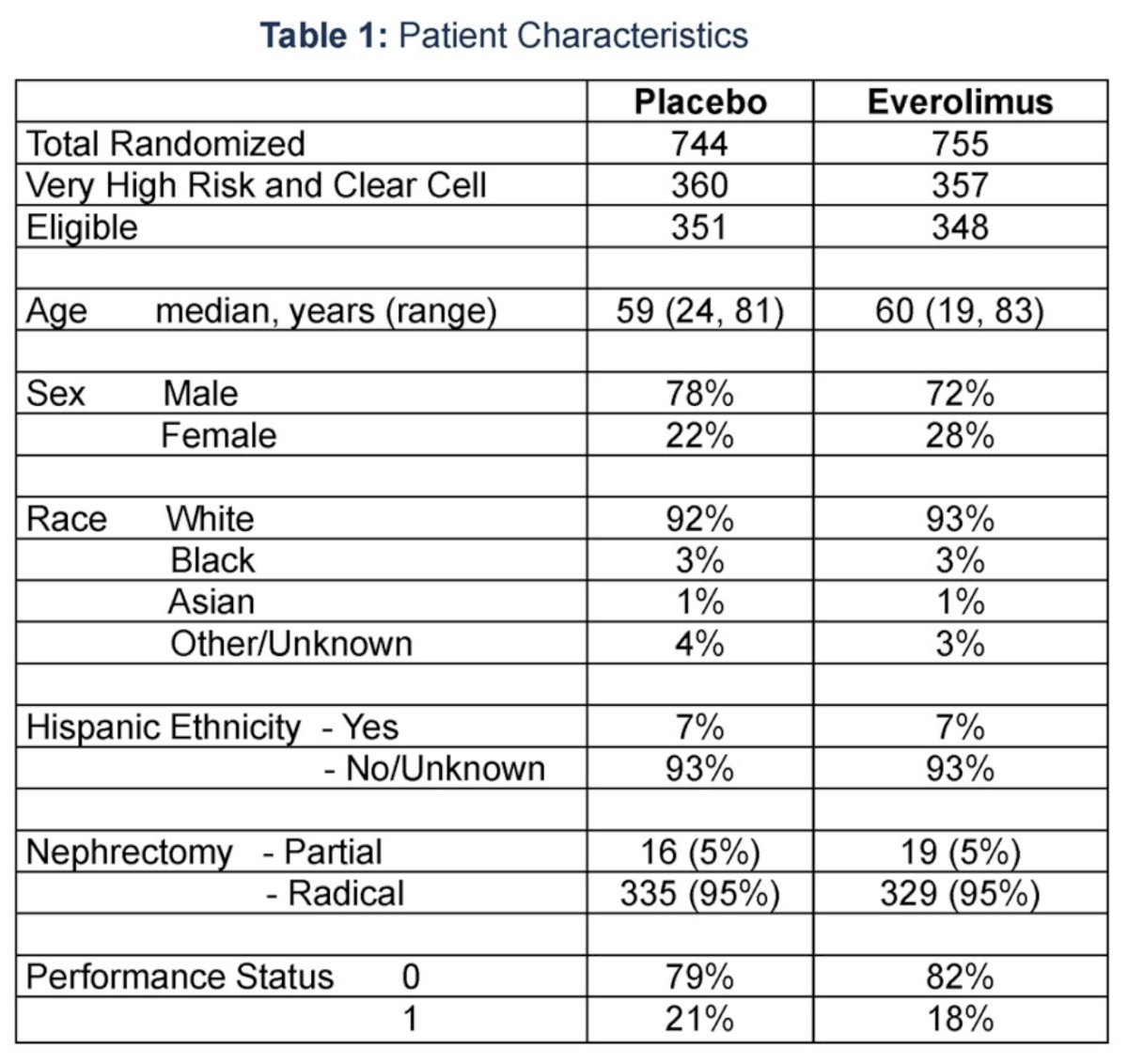
Of the 1,499 trial patients, 717 had both clear cell histology and very-high risk disease, 699 of whom were study eligible (everolimus: 348; placebo: 351).
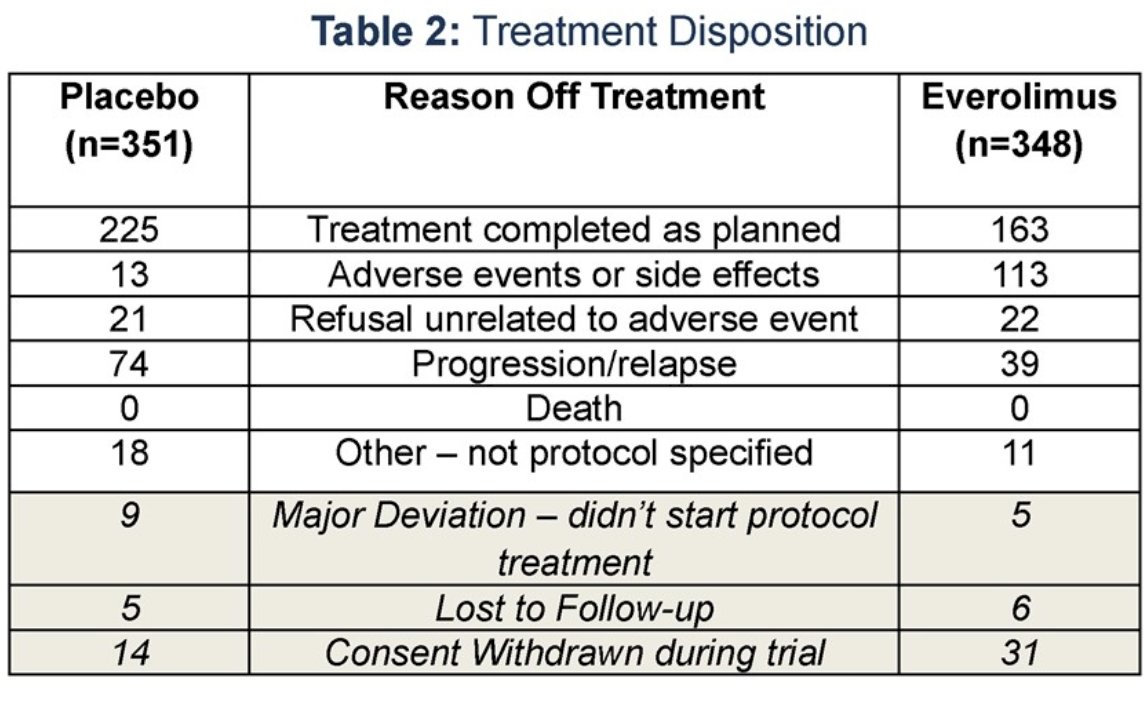
47% of patients in the everolimus arm completed all planned treatment, compared to 64% in the placebo arm.
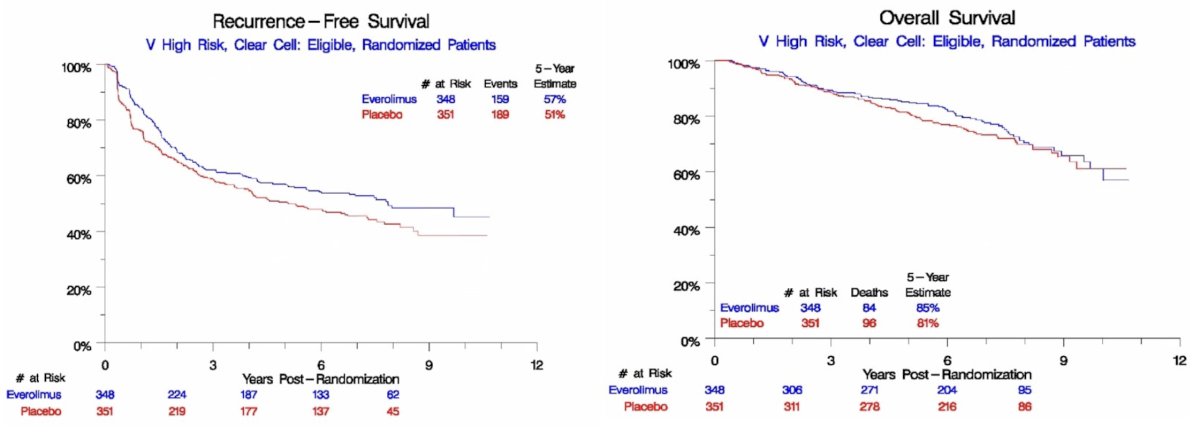
In the intent-to-treat cohort, there was evidence of an RFS benefit with everolimus (HR: 0.80, 95% CI: 0.65 – 0.99; 5-year survival: 57% versus 50%, p=0.040). However, no significant overall survival benefit was observed (HR: 0.85, 95% CI: 0.64 – 1.14, 5-year survival: 85% versus 81%, p=0.28). Grade 3 or worse adverse events occurred more frequently with everolimus (42% versus 8% for placebo).
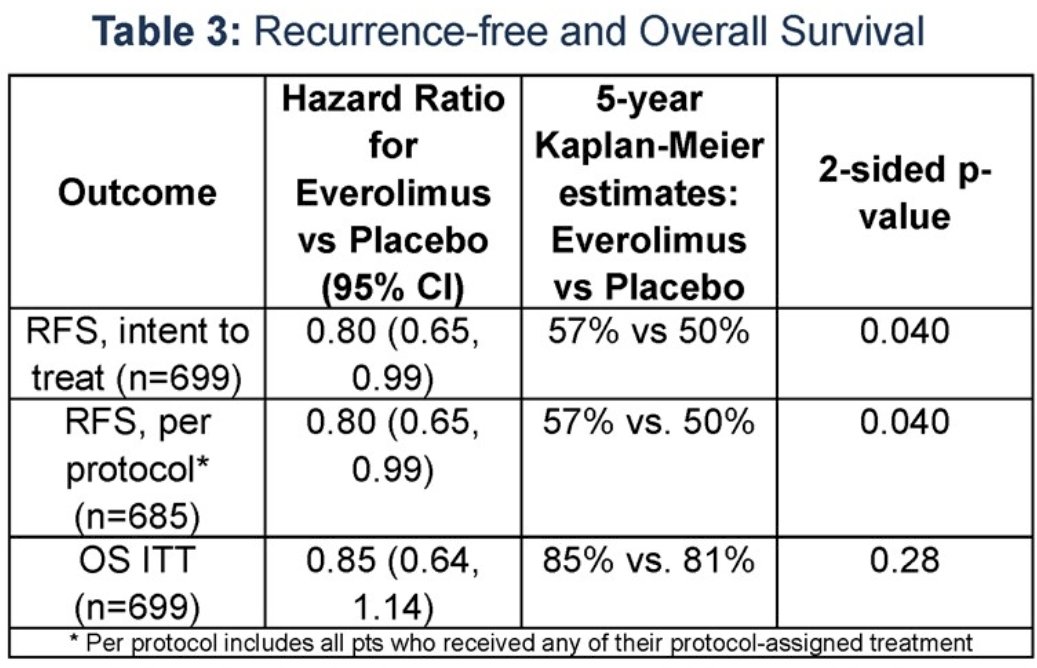
Dr. Lara concluded that for patients with clear cell RCC at very high-risk for recurrence, adjuvant everolimus was associated with significantly improved recurrence-free survival, compared to placebo, in a subset of patients comparable to those in S-TRAC and KEYNOTE-546. These results can inform the design and development of future adjuvant trials in high-risk RCC.
Presented by: Primo Lara, MD, Professor, Hematology and Oncology, University of California, Davis, CA
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:

