(UroToday.com) The 2023 ESMO annual meeting included a session on kidney cancer, featuring a presentation by Dr. Viktor Gruenwald discussing a post hoc analysis of the CLEAR trial assessing tumor response by baseline metastases in patients with renal cell carcinoma treated with lenvatinib + pembrolizumab versus sunitinib.
Lenvatinib + pembrolizumab is a standard of care in first line RCC based on significantly improved efficacy versus sunitinib in the CLEAR study.1 At the 2023 ESMO annual congress, Dr. Gruenwald and colleagues reported tumor response by baseline metastases at the final prespecified overall survival analysis time point of July 31, 2022. As follows are the criteria and results from the final prespecified OS analysis:
Treatment-naive adults with RCC were randomized to lenvatinib (20 mg orally daily in 21-day cycles) plus pembrolizumab (200 mg IV every 3 weeks) or lenvatinib (18 mg orally daily in 21-day cycles) plus everolimus (5 mg orally daily in 21-day cycles) or sunitinib (50 mg orally daily; 4 weeks on/2 weeks off). This post hoc analysis examined response by baseline metastases in lenvatinib + pembrolizumab (n=355) vs sunitinib (n=357) arms. Objective response rate (ORR), complete response rate, partial response rate, and progression-free survival (PFS) via independent imaging review per RECIST v1.1 by baseline metastatic characteristics:
- Site of metastasis: lung, lymph node, bone, liver, brain
- Number of metastatic sites: 1 vs ≥2
- Sums of diameters of target lesions: ≥60 mm vs <60 mm
The most common metastatic site by independent imaging review at baseline was the lung (lenvatinib + pembrolizumab: 71.0%; sunitinib: 63.9%). Overall, 22.5% of patients in the lenvatinib + pembrolizumab group and 24.9% of patients had bone metastases. Overall, 65.1% of patients randomized to lenvatinib + pembrolizumab and 66.1% of patients randomized to sunitinib had metastases at ≥2 sites.
ORR favored lenvatinib + pembrolizumab irrespective of metastatic site. ORR also favored lenvatinib + pembrolizumab in patients with 1 (OR 2.98 95% CI 1.71 to 5.21) or ≥2 metastatic sites (OR 5.06, 95% CI 3.40 to 7.53), and in patients with target lesion diameter sums ≥60 mm (OR 10.50, 95% CI 6.08 to 18.13) or <60 mm (OR 3.14, 95% CI 1.94 to 5.07). PFS clearly favored lenvatinib + pembrolizumab vs sunitinib across subgroups:
The median duration of response was generally longer with lenvatinib + pembrolizumab vs sunitinib across all subgroups:
Greater depth and breadth of tumor shrinkage at nadir was observed in target lesions in specific organ sites for patients in the lenvatinib + pembrolizumab arm. For example, the percentage change in sum of diameters of target lesions in lung from baseline to post-baseline nadir is as follows: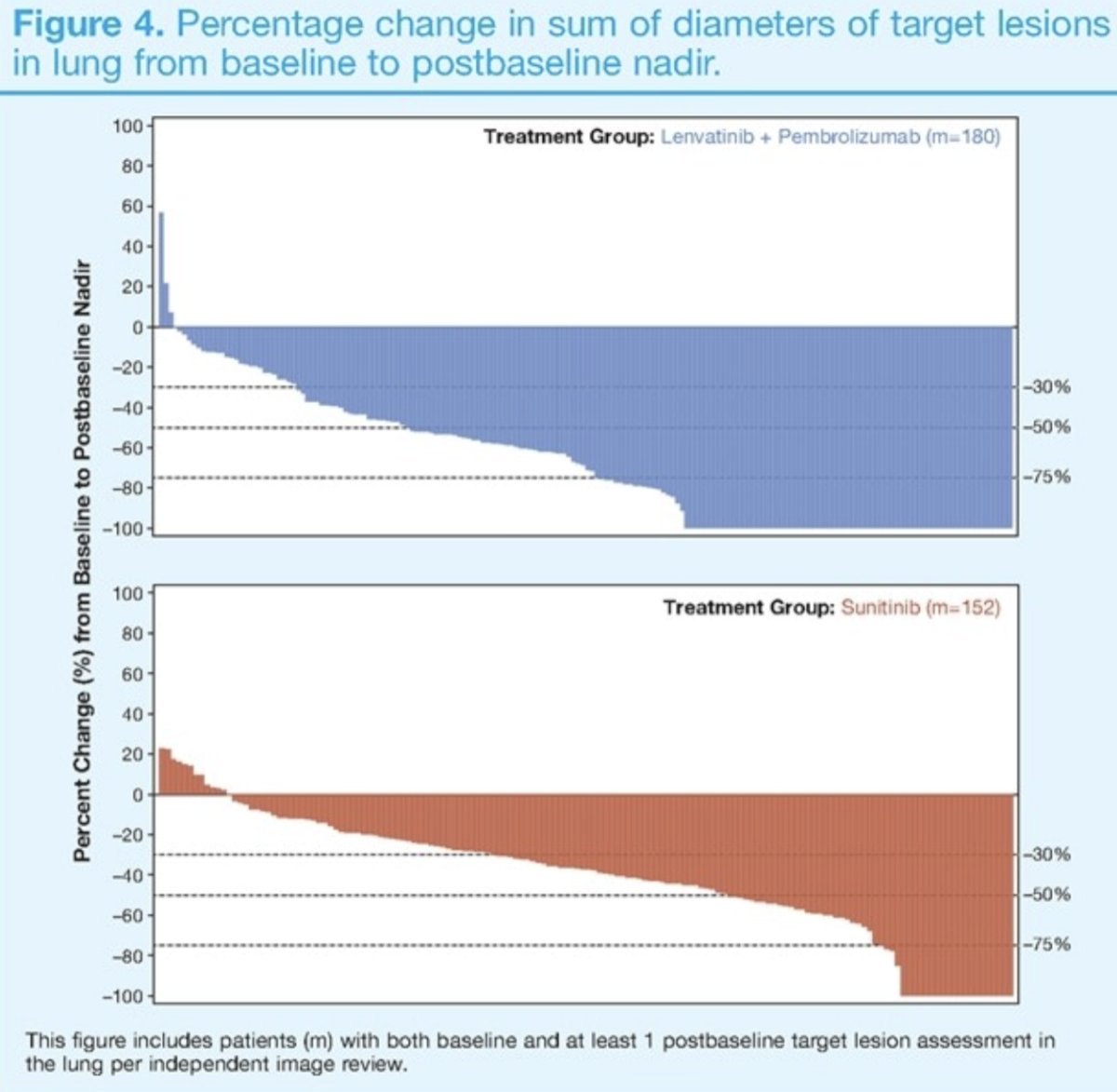
Dr. Gruenwald concluded his presentation discussing a post hoc analysis of the CLEAR trial assessing tumor response by baseline metastases in patients with renal cell carcinoma treated with lenvatinib + pembrolizumab versus sunitinib with the following concluding statements:
- Lenvatinib + pembrolizumab showed a clinically relevant efficacy across all subgroups of interest
- The median duration of response was generally longer with lenvatinib + pembrolizumab in all subgroups of interest
- Greater depth and breadth of tumor shrinkage was observed across organ sites for patients lenvatinib + pembrolizumab arm versus sunitinib arm
- Results of this post hoc analysis further support the early, deep, and durable tumor response benefit with lenvatinib + pembrolizumab versus sunitinib observed in the CLEAR trial
Presented by: Viktor Gruenwald, MD, PhD, University Hospital Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
Reference:


