(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. William Kelly discussing interim results from a phase 1 study of AMG 509 (xaluritamig) in patients with metastatic castration-resistant prostate cancer (mCRPC).
STEAP1 is a cell surface antigen highly expressed in prostate cancer and is associated with poor survival. Xaluritamig is a novel bispecific XmAb® 2+1 T-cell engager with two STEAP1 binding sites designed to facilitate T-cell–mediated lysis of STEAP1-expressing cells:
In preclinical studies, xaluritamig showed broad anti-cancer effects in prostate cancer xenograft models. At the 2023 ESMO annual meeting, Dr. Kelly and colleagues reported results from the dose exploration of xaluritamig monotherapy in a global, first-in-human, open-label study for patients with mCRPC.
Eligible patients had mCRPC refractory to prior novel hormonal therapy and 1–2 taxane regimens, ECOG 0–1, and adequate organ function. Xaluritamig was administered as an IV weekly or every 2 weeks with various dose levels/schedules. The study objectives were to evaluate safety, tolerability, antitumor activity, pharmacokinetics, and determine the maximum tolerated dose and recommended phase 2 dose. The trial design is as follows: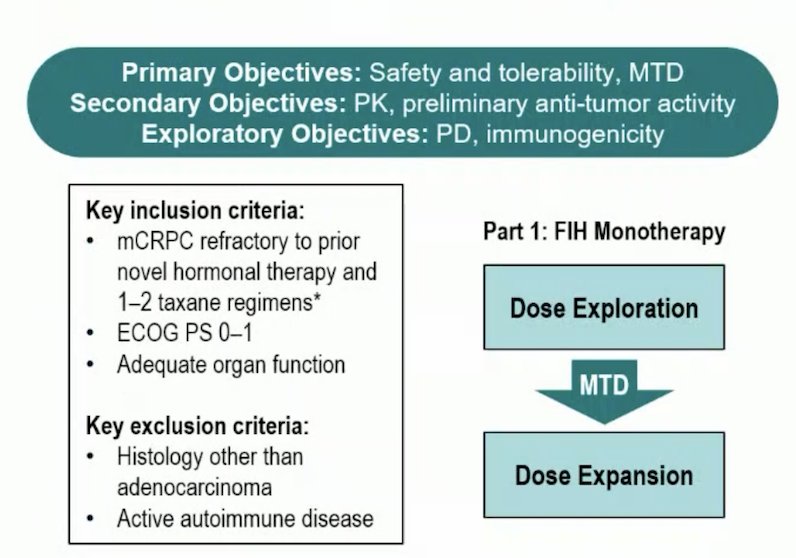
As of March 23, 2023, 97 patients in 15 dose levels received ≥1 dose of xaluritamig. The median age was 67 (range: 40–86) years and 67 patients (69.1%) had received > three prior lines of therapy, with a median of four prior lines of therapy. The baseline characteristics are as follows:

The dose exploration with step-dosing and prophylactic regimen to determine the maximum tolerated dose is as follows:
The maximum tolerated dose was identified as 1.5 mg IV weekly (3-step, D1 0.1 mg / D8 0.3 mg / D15 1.0 mg / D22+ 1.5 mg).
Treatment emergent adverse events were reported in 100% of patients (grade ≥3, 76%), and 97% reported treatment-related adverse events (grade ≥3, 55%). Treatment-related adverse events leading to discontinuation occurred in 19% of patients.: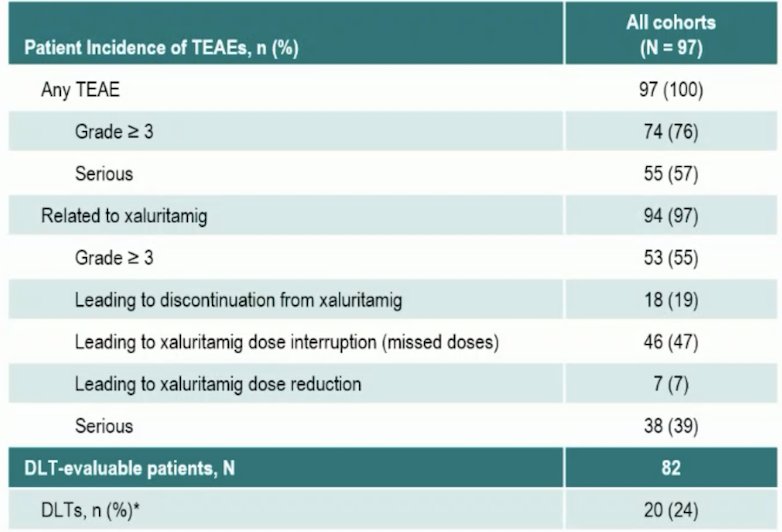
The most common adverse events were cytokine release syndrome (72.2%), fatigue (52.6%), anemia (45.4%), pyrexia (40.2%), and myalgia (39.2%). Cytokine release syndrome was primarily grade 1 or 2, one event being grade 3 (no grade 4/5 cytokine release syndrome events):
Preliminary pharmacokinetics showed dose-proportional increase in exposure with a mean terminal half-life of approximately 3-4 days. Cohorts 7b-13 (>= 0.75 mg target dose) had Ctrough values above predicted minimum efficacious exposure.
PSA50 (≥ 50% PSA decline) responses occurred in 43 patients (49%) and PSA90 (≥ 90% PSA decline) in 24 patients (28%). PSA responses were more frequent at higher dose levels (DL7b–15) than in lower dose levels (DL1–7a):
Overall, RECIST responses included 16 (24%) confirmed partial responses and 32 (48%) with stable disease. At higher dose levels, 15 patients (41%) had confirmed partial responses, and 14 (38%) stable disease:
Furthermore, 19 patients from high dose cohorts (n = 52) remained on treatment at data cutoff, and of those 13 patients remained on treatment for > 6 months. The median duration of response was 9.2 months (range: 1.9+ to 17.7+).
Anti-tumor activity has been observed against both soft tissue and bone disease:
Dr. Kelly concluded his presentation discussing interim results from a phase 1 study of xaluritamig in patients with mCRPC with the following take-home points:
- Xaluritamig is the first clinical T cell engager targeting STEAP1
- The maximum tolerated dose was established utilizing step-dosing and premedication
- The safety profile was clinically manageable with cytokine release syndrome that was generally low grade and primarily in cycle 1
- There was encouraging antitumor activity in heavily pre-treated men with mCRPC
- Dose expansion and optimization is currently ongoing to advance further development of xaluritamig as both a monotherapy and in combination
Presented by: William Kelly, DO, Jefferson Health, Philadelphia, PA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


