(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Praful Ravi discussing an individual patient data analysis of RCTs from the ICECaP consortium, specifically refining risk stratification in patients undergoing radiotherapy and long-term ADT for high-risk/locally advanced prostate cancer. Radiotherapy + long term ADT (18-36 months) is a standard-of-care in the treatment of high-risk/locally advanced prostate cancer. Furthermore, the addition of two years of abiraterone to radiotherapy + ADT has been shown to improve MFS and OS in selected men. However, efforts are ongoing to evaluate the intensification of systemic therapy beyond ADT to further improve outcomes. At the 2023 ESMO annual meeting, Dr. Ravi and colleagues evaluated 5-year MFS rates in subgroups of patients with high-risk/locally advanced prostate cancer to define patients more likely to benefit from treatment intensification, and guide design and interpretation of adjuvant trials in high-risk/locally advanced prostate cancer.
Individual patient data was assessed and pooled from patients with high-risk/locally advanced prostate cancer, as defined by any of the following 3 risk factors: Gleason ≥8, ≥cT3, PSA >20 ng/mL, or cN1, treated with radiotherapy + long term ADT in RCTs collated by ICECaP. The end points were MFS, time to metastasis, prostate cancer specific mortality, and OS. The 5-year outcomes were calculated by Kaplan-Meier method in various risk groups and by the number of risk factors. Multivariable Cox regression estimated hazard ratios for the 3 risk factors and cN1 disease, stratified by trials and years of enrolment.
There were 3,604 patients with high-risk/locally advanced prostate cancer treated with radiotherapy + long term ADT in 10 randomized trials between 1987 and 2016 that were eligible. The complete flow diagram for patient inclusion is as follows:
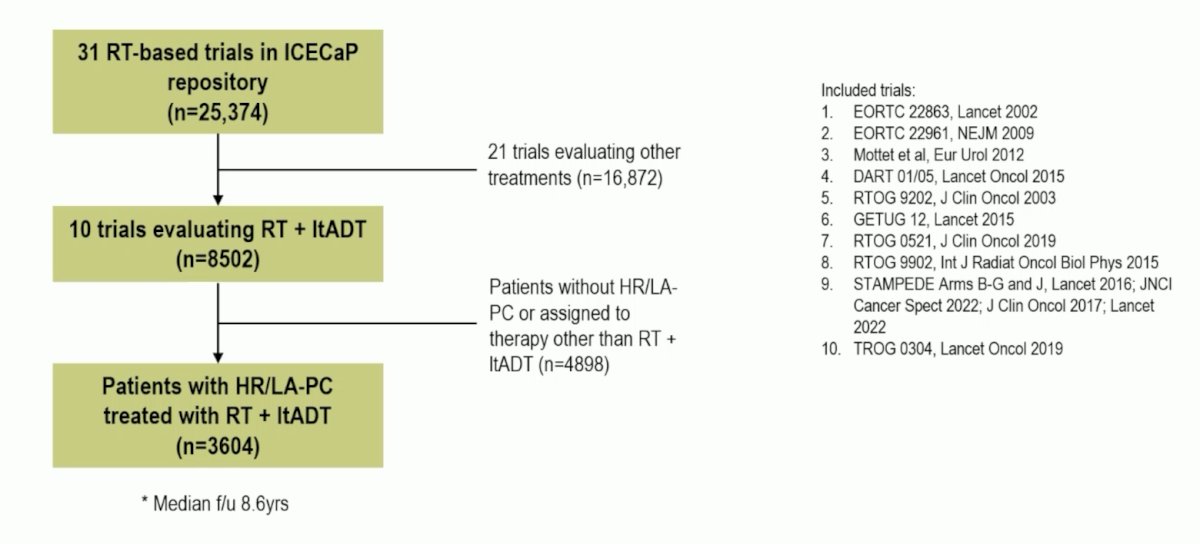
The median age was 68 (IQR 63-73) and median PSA was 24 (IQR 12-48) ng/mL, 2,602 (72%) were cT3/4, 1,942 (54%) had Gleason 8-10 disease, and 422 patients (12%) had cN1 disease. The HR for MFS was 1.52 (95% CI 1.35-1.70) for Gleason ≥8, 1.32 (1.08-1.61) for PSA >= 20 ng/mL, 1.22 (1.08-1.39) for cT3/T4, and 1.78 (1.49-2.13) for cN1. The complete predictors of outcomes from the multivariable models are as follows:

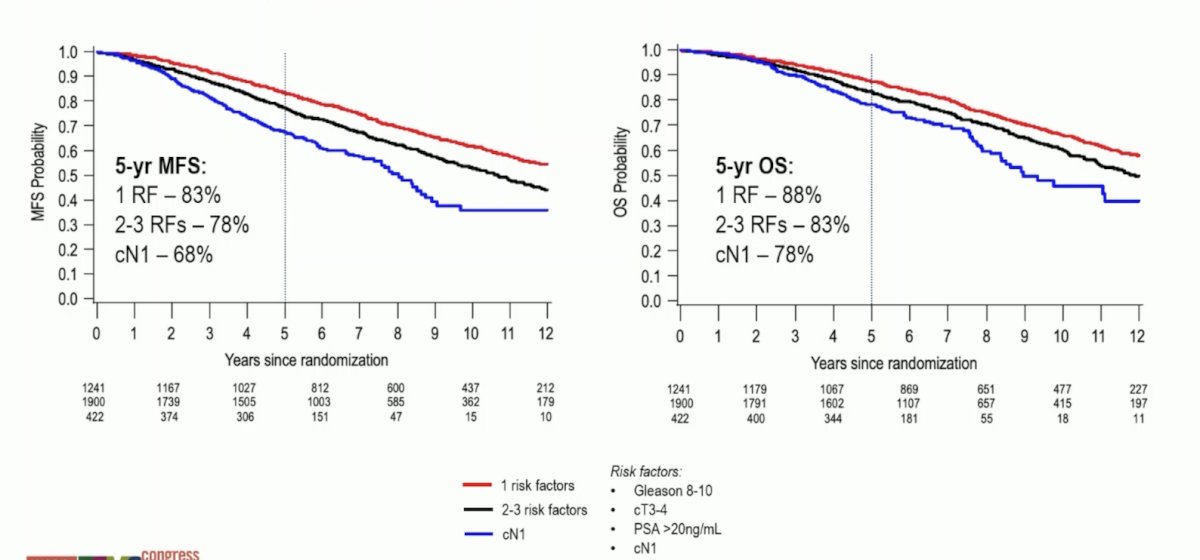
The 5-year MFS rate by risk groups is as follows:

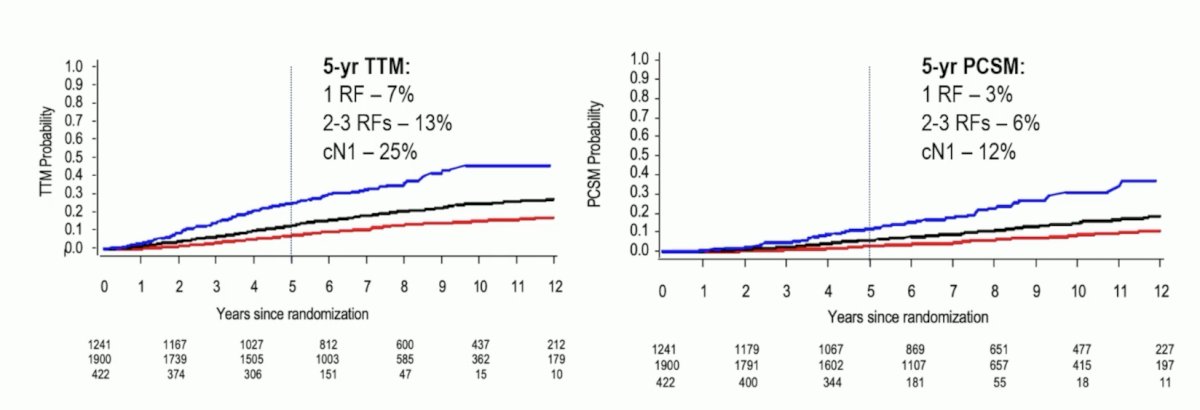
Both 5-year MFS and 5-year OS rates varied by risk groups, but most notably were worse for patients with cN1 disease:
Dr. Ravi concluded his presentation by discussing an individual patient data analysis of RCTs from the ICECaP consortium, specifically refining risk stratification in patients undergoing radiotherapy and long-term ADT for high-risk/locally advanced prostate cancer with the following take-home points:
- Gleason score, clinical T/N stage, and PSA have independent prognostic value in patients with high-risk/locally advanced prostate cancer treated with radiotherapy + long term ADT
- Long-term outcomes are clearly differentiated by the overall number of risk factors present at baseline
- Patients with 2 or 3 risk factors, or cN1 disease had 5-year MFS rates of <80% and are therefore most likely to benefit from treatment intensification beyond radiotherapy and long term ADT
- These findings will guide patient counseling and provide the framework to aid in interpretation of adjuvant trials in high-risk/locally advanced prostate cancer
Presented by: Praful Ravi, MB, BChir, MRCP, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.


