(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a discussant presentation by Dr. Daniel Heinrich. For this discussant presentation, Dr. Heinrich discussed the abstract “Refining risk stratification in patients undergoing radiotherapy and long-term ADT for high-risk/locally advanced prostate cancer: An individual patient data analysis of RCTs from the ICECaP consortium” presented by Dr. Praful Ravi, and “ENZA-p (ANZUP 1901): Enzalutamide and 177Lu-PSMA-617 in poor-risk mCRPC, a randomized, phase 2 trial” presented by Dr. Louise Emmett.
Dr. Heinrich started by highlighting that combination therapies, specifically the triplet therapy of ADT + darolutamide + docetaxel in the ARASENS trial1 have impressed us in the metastatic hormone sensitive prostate cancer disease space. Additionally, we have been impressed by combination therapies, specifically olaparib + abiraterone in the PROpel trial,2 for metastatic castration resistant prostate cancer. But, where might this treatment intensification end? Dr. Heinrich notes that with non-overlapping toxicities, even 5, 6, or 7 drug regimens may be feasible, but they definitely come at a cost of quality of life and in the typically elderly and comorbid prostate cancer population at non-neglectable risk of serious complications. Additionally, these patients represent an increased burden for healthcare systems, both financially and due to increased follow-up frequency and adverse event handling.
With regards to the ICECaP study assessing risk stratification of patients undergoing radiotherapy + long term ADT for high risk/locally advanced prostate cancer, Dr. Heinrich notes that this study does assist us in ascertaining who may benefit from treatment intensification. This study found that the hazard ratio for MFS was 1.52 (95% CI 1.35-1.70) for Gleason ≥8, 1.32 (1.08-1.61) for PSA >= 20 ng/mL, 1.22 (1.08-1.39) for cT3/T4, and 1.78 (1.49-2.13) for cN1. Additionally, the 5-year MFS risk groups do stratify patients based on low, medium, and high risk for developing metastases:
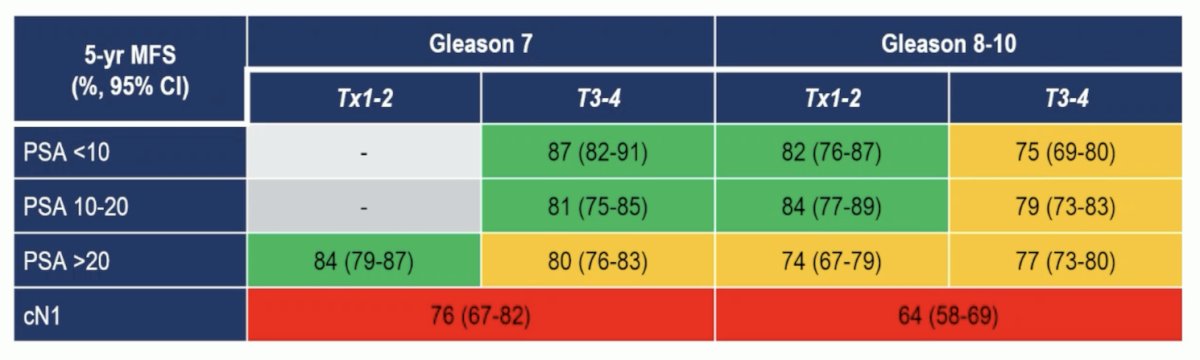
Both 5-year MFS and 5-year OS rates varied by risk groups, but most notably were worse for patients with cN1 disease:

Based on the results from the ICECaP study, Dr. Heinrich offered the following conclusions and future perspectives:
- There is an incredible wealth of knowledge to be gleaned from individual patient data analyses
- The study provides a very easy way to assess stratification based on regularly collected and easily collectable baseline disease characteristics
- The N1 subgroup in this study, which seems to have the greatest potential benefit from treatment intensification, was defined based on conventional imaging and represents only 12% of the total population
- How would these numbers look like and would the results be impacted if all patients been staged with PSMA PET/CT and N+ status defined based on molecular imaging?
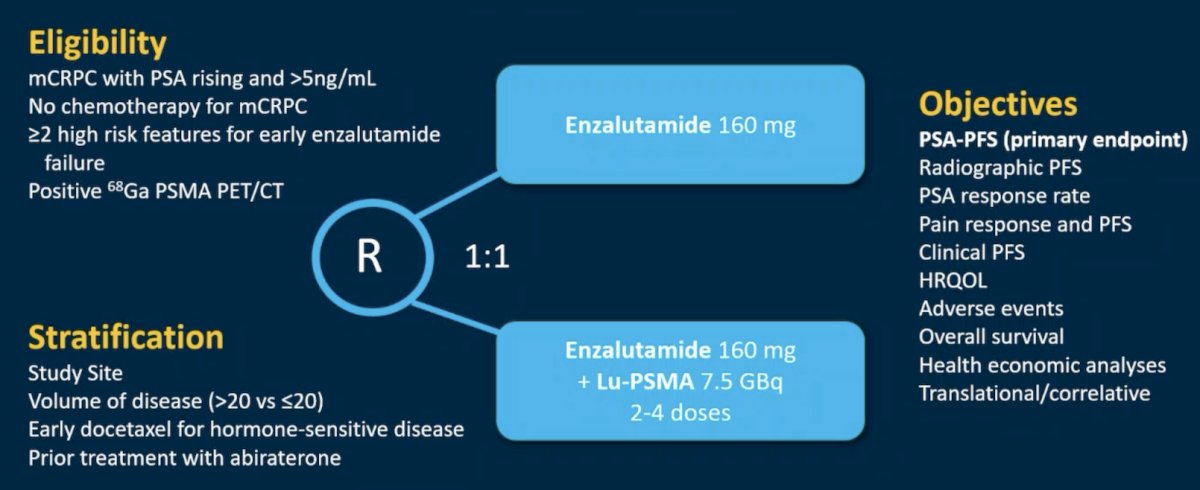
Dr. Heinrich then discussed the ENZA-p trial, which included mCRPC patients without prior chemotherapy and those with >=2 high risk features for early enzalutamide failure. Patients were randomized (1:1) to either enzalutamide 160 mg daily (enzalutamide-alone) or enzalutamide 160 mg daily plus adaptive dosing LuPSMA 7.5 GBq on days 15 and 57, with 2 further doses of LuPSMA given if there was persistent PSMA-positive disease on interim 68Ga-PSMA PET (day 92) (enzalutamide + LuPSMA). Stratification was by study site, volume of disease, early docetaxel for hormone sensitive disease, and prior treatment with abiraterone. The trial design for ENZA-p is as follows:
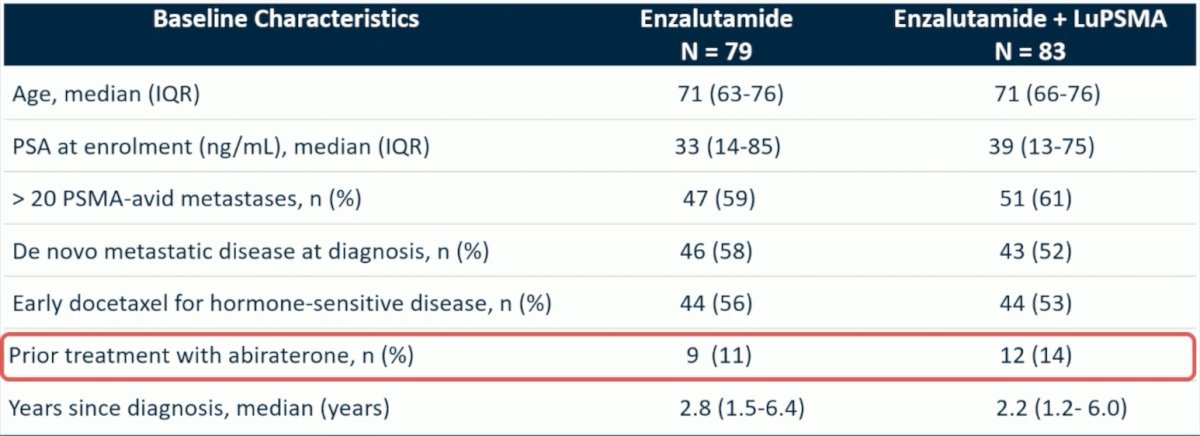
Of note, there were 11% of patients in the enzalutamide arm alone and 14% of patients in the enzalutamide + LuPSMA arm that had previously been treated with abiraterone:
We know that previous trials have looked at new hormonal agent switches:
- The PROfound control arm3 showed a response rate of 2.3% and rPFS of 3.55 months
- The CARD control arm4 showed a response rate of 11.5% and PFS of 2.7 months
- The PLATO trial (enzalutamide + abiraterone or placebo + abiraterone after progression on enzalutamide): showed a time to progression of 2.8 months in both arms
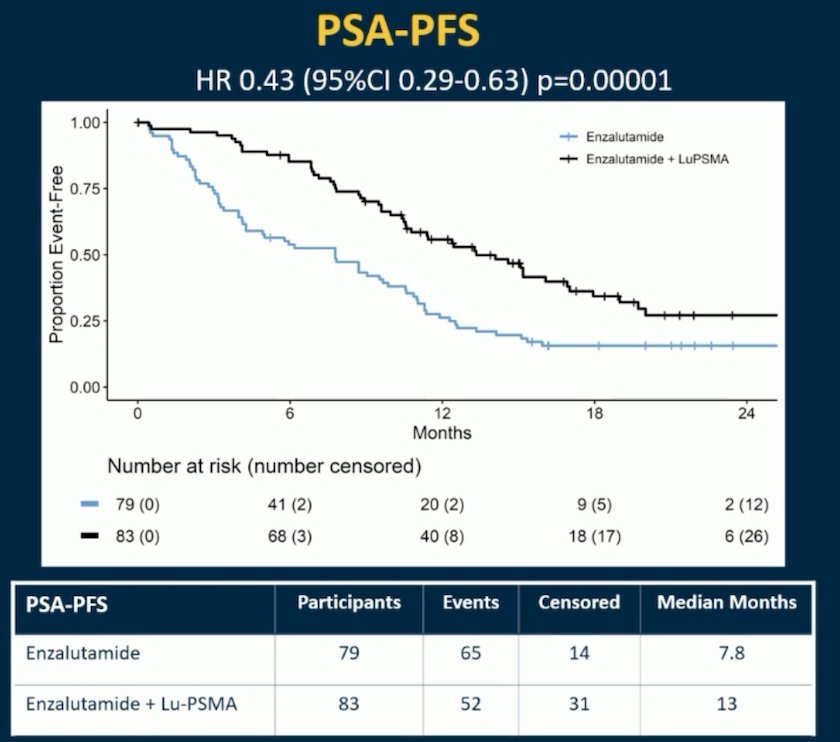
Thus, new hormonal agent switch is not recommended and therefore not acceptable as a control arm treatment, but fortunately, few patients in ENZA-p had a hormonal switch, which is unlikely to affect the outcome. In ENZA-p, over a median follow up of 20 months (IQR 18-21), PSA-PFS was longer with enzalutamide + LuPSMA vs enzalutamide-alone (median 13 vs 7.8 months; HR 0.43, 95% CI 0.29-0.63, p<0.001):
Symptomatic adverse events were reported in 33% (27/81) of patients assigned enzalutamide + LuPSMA vs 35% (28/79) enzalutamide-alone. As follows is a tornado plot summarizing the adverse events of interest:

Dr. Heinrich offered the following conclusions and future perspectives based on data from the ENZA-p trial:
- The correct study design for a first-line mCRCP study is standard of care versus standard of care + intervention, in a well chosen study population
- Adaptive dosing is an important additional feature and any endpoints specific to this (ie. health economics or second PFS after a possible re-challenge with LuPSMA) will be very interesting to follow
- Enzalutamide + LuPSMA appears to be a feasible combination
- More is obviously better for some patients, but selection is the key
- But, all approved mCRPC treatment regimens are based on an OS advantage, thus this study needs longer follow-up for secondary endpoints
Presented by: Daniel Heinrich, MD, Brumunddal, Norway
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med 2019 Dec 26;381(26):2506-2518.
ESMO 2023: Enzalutamide and 177Lu-PSMA-617 in Poor-Risk Metastatic Castration-Resistant Prostate Cancer (mCRPC), a Randomized, Phase 2 Trial
ESMO 2023: Refining Risk Stratification in Patients Undergoing Radiotherapy and Long-Term ADT for High-Risk/locally Advanced Prostate Cancer: An Individual Patient Data Analysis of RCTs from the ICECaP Consortium


