(UroToday.com) The 2023 ESMO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Anne-Laure Giraudet discussing descriptive results of the first real-life data on 177Lu-PSMA-617 from the early access program in France. 177Lu-PSMA-617 is a radiopharmaceutical with binding affinity to PSMA, which is expressed in 90% of mCRPC.
The VISION trial showed that 177Lu-PSMA-617 added to the standard of care, prolonging imaging based progression-free survival and overall survival in patients with PSMA-positive mCRPC.1 Thus, cohort temporary authorization for use was granted to 177Lu-PSMA-617 by French Health Authorities for patients with this indication. This early access program began on December 1, 2021, and is still in progress.
PSMA positive patients with mCRPC pretreated with 1-2 taxane chemotherapy and ≥1 androgen receptor pathway inhibitor were included. Treatment administration planned six IVs of 177Lu-PSMA-617 (7.4 GBQ) every 6 weeks. Patient characteristics, efficacy, and safety data were collected during the six follow-up visits (average: 17.1 ± 11.2 weeks). Patient characteristics and safety data were described from the total patient population included in this early access program.
From December 1, 2021, to June 30, 2023, 945 patients were included in this early access program. The baseline characteristics of these patients, and a side by side comparison to the VISION trial patients, are as follows:
Compared to patients in the VISION trial, patients from the early access cohort were older, with poorer performance status, and a high prevalence of lymph node metastases. Additionally, a higher proportion also received 2 or more next generation hormonal agents and 2 taxane chemotherapies compared to VISION trial patients: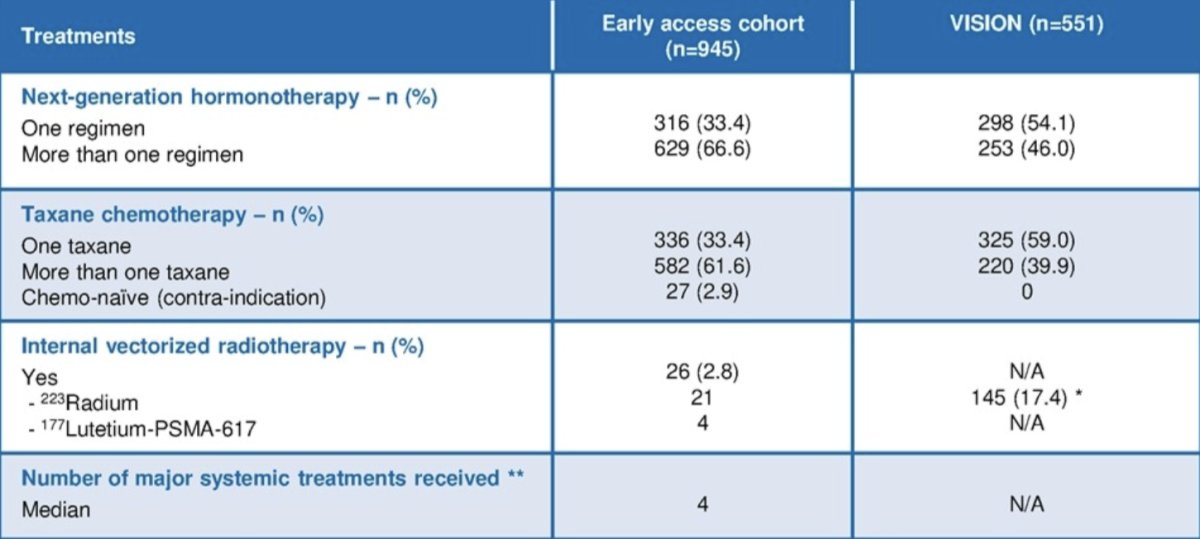
Of these 945 patients, 749 patients received a concomitant treatment as summarized below: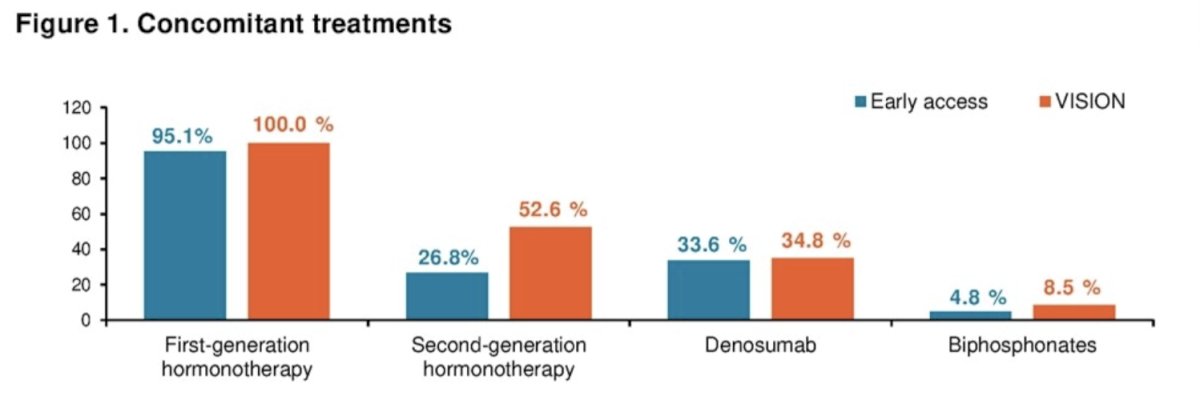
The efficacy data were assessed from 535 patients that were included from December 1, 201 to December 1, 2022. Among these 535 patients, 119 were still undergoing treatment, and patients received a median of 4 cycles in comparison to the median of 5 in the VISION trial. Radiological follow-up was performed according to investigator’s choice and in most were assessed by CT scan + scintigraphy, but in some cases by PET/CT follow-up. Median imaging based PFS was 6.8 (range: 5.9-7.8) months:
Overall, 5.2% of patients have never reported stabilization or improvement of symptoms and had an immediate progression that never improved:
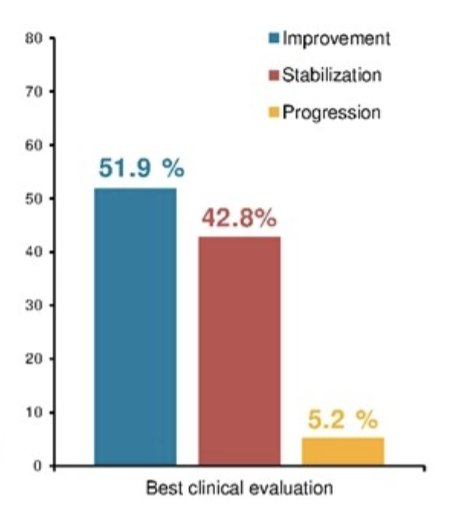
Additionally, for 18% of patients, there was no drop or stabilization in PSA observed:

Treatment related adverse events were as expected and consistent with those described in the VISION trial. Despite altered general condition compared to VISION patients, safety data were similar. The most frequently reported adverse event was hematotoxicity with no new safety signals:
Dr. Giraudet concluded her presentation discussing descriptive results of the first real-life data on 177Lu-PSMA-617 from the early access program in France with the following concluding statements:
- Compared to patients from the VISION trial, patients in this early access cohort from France had poorer performance status, and higher prevalence of lymph node metastases, in addition to being heavily pretreated
- Patients have received a median of 4 177Lu-PSMA-617 treatments, with only 5.2% of patients never experiencing stabilization or improvement in symptoms and 18% not experience a decrease in PSA
- Despite this comorbid population compared to the VISION trial, safety data were comparable and there were no unexpected adverse events reported
Presented by: Anne-Laure Giraudet, MD, Centre Léon Bérard, Lyon, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:


