(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a new diagnostic tools abstracts poster session. Dr. Xin Ye presented the results of a study evaluating a novel next generation sequencing (NGS) assay for the detection of homologous recombination repair (HRR) gene alterations in prostate cancer.
Homologous recombination comprises a series of interrelated pathways that function in the repair of DNA double-stranded breaks and inter-strand crosslinks. Accordingly, germline (i.e., inherited) HRR gene alterations, including BRCA1/2 and ATM mutations, predispose patients to an increased risk of future prostate cancer development and adverse outcomes when mutated in the germline or somatic settings. Since 2020, numerous poly ADP ribose polymerase (PARP) inhibitors have been US Food and Drug Administration (FDA) approved for the treatment of patients with germline or somatic HRR mutations:
- Rucaparib in May 2020 for mCRPC patients with deleterious Breast Cancer (BRCA) mutations who had been previously treated with an ARPI and taxane-based chemotherapy1 (TRITON2)2
- Olaparib in May 2020 for patients with deleterious or suspected homologous recombination repair (HRR) gene-mutated mCRPC with progression following prior abiraterone or enzalutamide3 (PROfound4)
- Olaparib in combination with abiraterone and prednisone (or prednisolone) in May 2023 for mCRPC patients with deleterious or suspected deleterious BRCA mutations5 (PROpel6)
- Talazoparib in combination with enzalutamide in June 2023 for mCRPC patients with HRR gene mutations7 (TALAPRO-2).8
Despite the importance of NGS testing for the detection of HRR gene alterations, the currently available commercial tests are limited and usually entail large NGS panels with high associated costs and long turnaround times. To overcome these limitations, a novel HANDLE (Halo-shape ANnealing and Defer-Ligation Enrichment) based NGS assay, AmoyDx HRD Complete, was established to evaluate single nucleotide variant, short insertion and deletion, and homozygous deletion of 17 HRR genes in prostate cancer tissues, with a turnaround time of 4 days.
The sensitivity of this assay was evaluated in serially diluted cell lines with varying DNA input:
- Gradient mutation allele frequency (7%, 5%, and 3%) for single nucleotide variant short insertion and deletion
- Different tumor content (50%, 40%, 30%, and 20%) for homozygous deletions
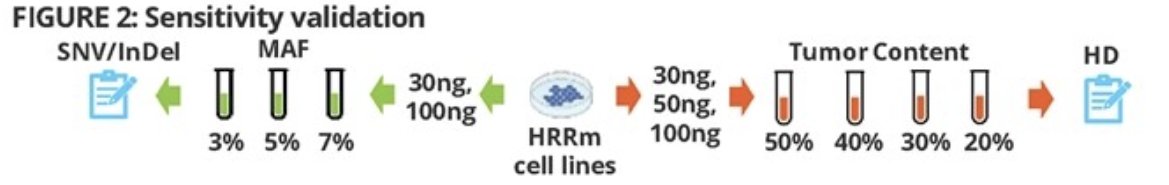
The specificity was validated in wild type cell lines. The testing success rate was evaluated using 100 formalin-fixed paraffin-embedded prostate cancer tissues (up to 10 years old). A “three-tube” library process was developed for samples which failed DNA, library, or sequencing quality control with standard processing. The accuracy was assessed in >200 formalin-fixed paraffin-embedded prostate cancer tissues, using commercially available AmoyDx HRD54 NGS kit (hybridization capture based) and AmoyDx BRCA1/2 NGS kit (CE-IVD) as reference tests.
The sensitivity of the assay for HRR single nucleotide variant short insertions and deletions was 99.4% (179/180) at a gradient mutation allele frequency of 5% with 30 ng DNA. The sensitivity for HRR homozygous deletions was 100% at the gene level with 30% tumor content and at the exon level with 40% tumor content with 100 ng DNA. The specificity was 100%.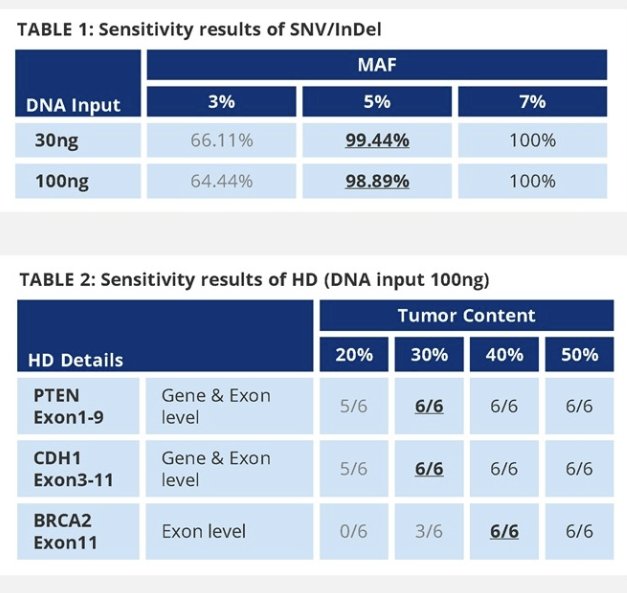
54/100 (54%) prostate cancer tissues had valid sequencing results using standard process, whereas the “three-tube” library improved the success rate to 84%.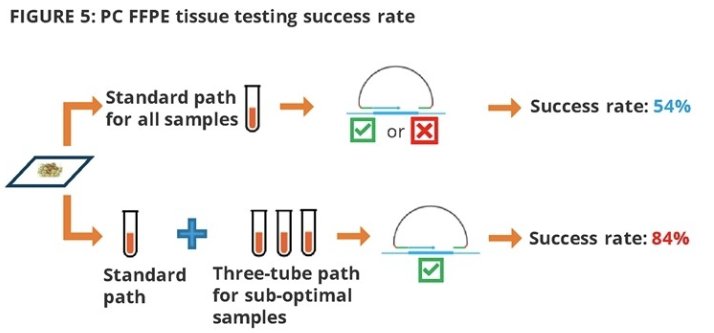
For HRR single nucleotide variant short insertions and deletions, 98/100 prostate cancer tissues showed concordant results with HRD54 kit:
- Positive percent agreement: 96.8%
- Negative percent agreement: 98.6%
- Overall percent agreement: 98%
For HRR homozygous deletion accuracy, 130/132 prostate cancer tissues showed concordant results with the HRD54 kit:
- Positive percent agreement: 100%
- Negative percent agreement: 98.4%
- Overall percent agreement: 98.5%
Specifically, the accuracy of BRCA1/2 single nucleotide variant short insertions and deletions was further verified with 100% concordance with the BRCA1/2 kit.
The authors concluded that given the high sensitivity and specificity, short turnaround time, and high success rate, the CE (conformite Europeenne) marked HRD Complete assay provides a robust and reliable test approach for HRR testing in prostate cancer patients, allowing for a personalized treatment approach.
Presented by: Xin Ye, MD, Shanghai, China
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:
- Anscher, M.S. et al. (2021) FDA approval summary: Rucaparib for the treatment of patients with deleterious BRCA-mutated metastatic castrate-resistant prostate cancer. The Oncologist.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- Center for Drug Evaluation and Research (2023) FDA approves Olaparib for HRR gene-mutated metastatic castration-resis, U.S. Food and Drug Administration.
- De Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091-2102.
- Center for Drug Evaluation and Research (2023 FDA D.I.S.C.O. burst: Lynparza, U.S. Food and Drug Administration.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evid. 2022;1(9).
- ESMO (2023) FDA approves Talazoparib with Enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer, ESMO.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291-303.


